Flame Ionisation Detector on:
[Wikipedia]
[Google]
[Amazon]
 A flame ionization detector (FID) is a
A flame ionization detector (FID) is a
 A flame ionization detector (FID) is a
A flame ionization detector (FID) is a scientific instrument
A scientific instrument is a device or tool used for scientific purposes, including the study of both natural phenomena and theoretical research.
History
Historically, the definition of a scientific instrument has varied, based on usage, laws, a ...
that measures analyte
An analyte, component (in clinical chemistry), or chemical species is a substance or chemical constituent that is of interest in an analytical procedure. The purest substances are referred to as analytes, such as 24 karat gold, NaCl, water, et ...
s in a gas stream. It is frequently used as a detector in gas chromatography
Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, ...
. The measurement of ion per unit time make this a mass sensitive instrument. Standalone FIDs can also be used in applications such as landfill gas monitoring
Landfill gas monitoring is the process by which gases that are collected or released from landfills are electronically monitored. Landfill gas may be measured as it escapes the landfill ("Surface Monitoring") or may be measured as it is collected ...
, fugitive emission
Fugitive emissions are leaks and other irregular releases of gases or vapors from a pressurized containment – such as appliances, storage tanks, pipelines, wells, or other pieces of equipment – mostly from industrial activities. In addition ...
s monitoring and internal combustion engine
An internal combustion engine (ICE or IC engine) is a heat engine in which the combustion of a fuel occurs with an oxidizer (usually air) in a combustion chamber that is an integral part of the working fluid flow circuit. In an internal co ...
emissions measurement in stationary or portable instruments.
History
The first flame ionization detectors were developed simultaneously and independently in 1957 by McWilliam and Dewar at Imperial Chemical Industries of Australia and New Zealand (ICIANZ, see Orica history) Central Research Laboratory, Ascot Vale,Melbourne
Melbourne ( ; Boonwurrung/ Woiwurrung: ''Narrm'' or ''Naarm'') is the capital and most populous city of the Australian state of Victoria, and the second-most populous city in both Australia and Oceania. Its name generally refers to a me ...
, Australia. and by Harley and Pretorius at the University of Pretoria
The University of Pretoria ( af, Universiteit van Pretoria, nso, Yunibesithi ya Pretoria) is a multi-campus public university, public research university in Pretoria, the administrative and de facto capital of South Africa. The university was ...
in Pretoria
Pretoria () is South Africa's administrative capital, serving as the seat of the executive branch of government, and as the host to all foreign embassies to South Africa.
Pretoria straddles the Apies River and extends eastward into the foot ...
, South Africa
South Africa, officially the Republic of South Africa (RSA), is the southernmost country in Africa. It is bounded to the south by of coastline that stretch along the South Atlantic and Indian Oceans; to the north by the neighbouring count ...
.
In 1959, Perkin Elmer Corp. included a flame ionization detector in its Vapor Fractometer.
Operating principle
The operation of the FID is based on the detection of ions formed during combustion of organic compounds in ahydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
flame
A flame (from Latin '' flamma'') is the visible, gaseous part of a fire. It is caused by a highly exothermic chemical reaction taking place in a thin zone. When flames are hot enough to have ionized gaseous components of sufficient density the ...
. The generation of these ions is proportional to the concentration of organic species in the sample gas stream.
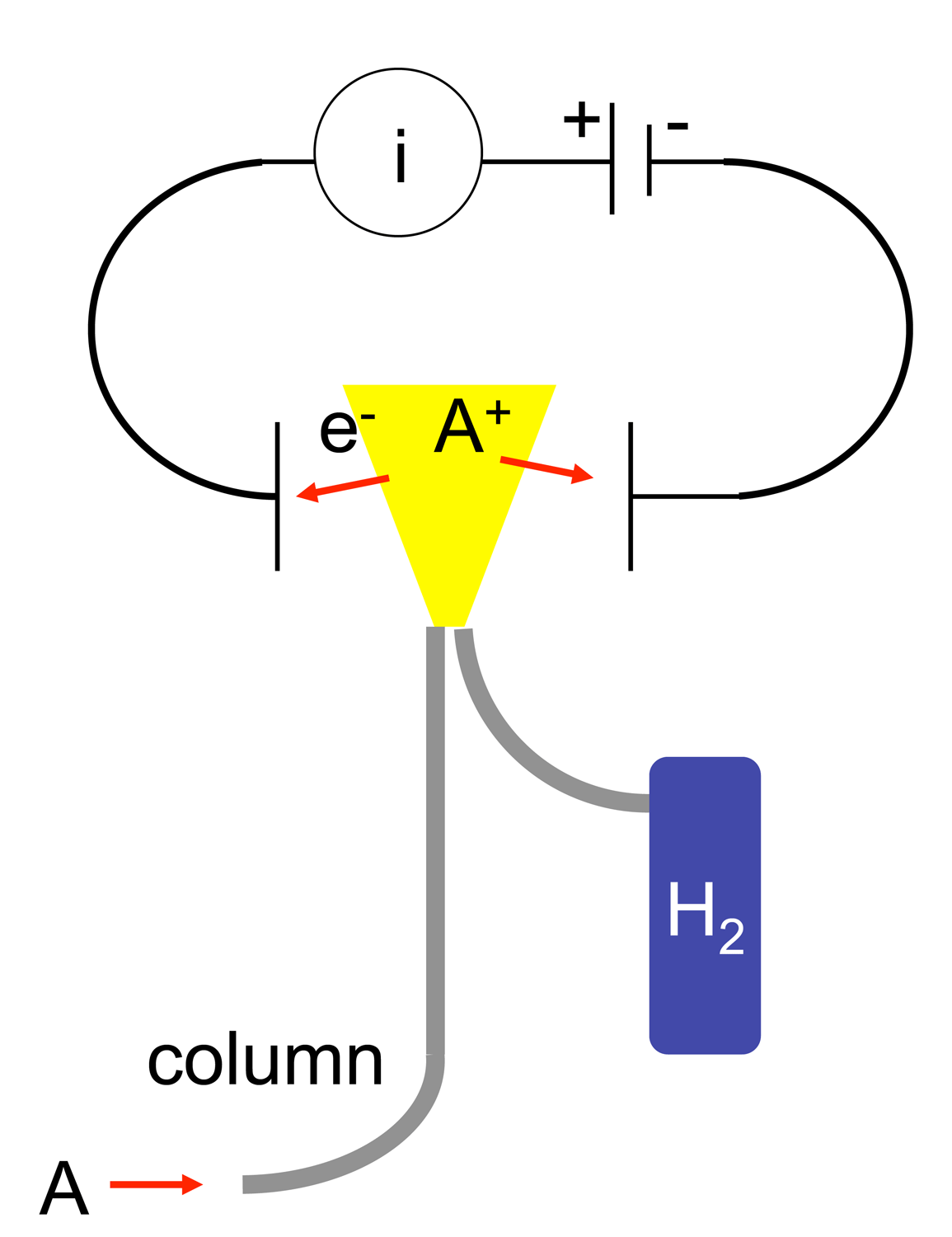
To detect these ions, two electrodes
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or air). Electrodes are essential parts of batteries that can consist of a variety of materials dep ...
are used to provide a potential difference. The positive electrode acts as the nozzle head where the flame is produced. The other, negative electrode is positioned above the flame. When first designed, the negative electrode was either tear-drop shaped or angular piece of platinum. Today, the design has been modified into a tubular electrode, commonly referred to as a collector plate. The ions thus are attracted to the collector plate and upon hitting the plate, induce a current. This current is measured with a high-impedance picoammeter and fed into an integrator
An integrator in measurement and control applications is an element whose output signal is the time integral of its input signal. It accumulates the input quantity over a defined time to produce a representative output.
Integration is an importan ...
. The manner in which the final data is displayed is based on the computer and software. In general, a graph is displayed that has time on the x-axis and total ion on the y-axis.
The current measured corresponds roughly to the proportion of reduced carbon atoms in the flame. Specifically how the ions are produced is not necessarily understood, but the response of the detector is determined by the number of carbon atoms (ions) hitting the detector per unit time. This makes the detector sensitive to the mass rather than the concentration, which is useful because the response of the detector is not greatly affected by changes in the carrier gas flow rate.
Response factor
FID measurements are usually reported "as methane," meaning as the quantity ofmethane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Ear ...
which would produce the same response. The same quantity of different chemicals produces different amounts of current, depending on the elemental composition of the chemicals. The response factor Response factor, usually in chromatography and spectroscopy, is the ratio between a signal produced by an analyte, and the quantity of analyte which produces the signal. Ideally, and for easy computation, this ratio is unity (one). In real-world sc ...
of the detector for different chemicals can be used to convert current measurements into actual amounts of each chemical.
Hydrocarbons generally have response factors that are equal to the number of carbon atoms in their molecule (more carbon atoms produce greater current), while oxygenates and other species that contain heteroatoms
In chemistry, a heteroatom () is, strictly, any atom that is not carbon or hydrogen.
Organic chemistry
In practice, the term is usually used more specifically to indicate that non-carbon atoms have replaced carbon in the backbone of the molecul ...
tend to have a lower response factor. Carbon monoxide
Carbon monoxide ( chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
and carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is t ...
are not detectable by FID.
FID measurements are often labelled "total hydrocarbons" or "total hydrocarbon content" (THC), although a more accurate name would be "total volatile hydrocarbon content" (TVHC), as hydrocarbons which have condensed out are not detected, even though they are important, for example safety when handling compressed oxygen.
Description
The design of the flame ionization detector varies from manufacturer to manufacturer, but the principles are the same. Most commonly, the FID is attached to a gas chromatography system. Theeluent
In analytical and organic chemistry, elution is the process of extracting one material from another by washing with a solvent; as in washing of loaded ion-exchange resins to remove captured ions.
In a liquid chromatography experiment, for exa ...
exits the gas chromatography column (A) and enters the FID detector’s oven (B). The oven is needed to make sure that as soon as the eluent exits the column, it does not come out of the gaseous phase and deposit on the interface between the column and FID. This deposition would result in loss of eluent and errors in detection. As the eluent travels up the FID, it is first mixed with the hydrogen fuel (C) and then with the oxidant (D). The eluent/fuel/oxidant mixture continues to travel up to the nozzle head where a positive bias voltage exists. This positive bias helps to repel the oxidized carbon ions created by the flame (E) pyrolyzing the eluent. The ions (F) are repelled up toward the collector plates (G) which are connected to a very sensitive ammeter, which detects the ions hitting the plates, then feeds that signal to an amplifier, integrator, and display system(H). The products of the flame are finally vented out of the detector through the exhaust port (J).
Advantages and disadvantages
Advantages
Flame ionization detectors are used very widely in gas chromatography because of a number of advantages. *Cost: Flame ionization detectors are relatively inexpensive to acquire and operate. *Low maintenance requirements: Apart from cleaning or replacing the FID jet, these detectors require little maintenance. *Rugged construction: FIDs are relatively resistant to misuse. *Linearity and detection ranges: FIDs can measure organic substance concentration at very low (10−13 g/s) and very high levels, having a linear response range of 107 g/s.Disadvantages
Flame ionization detectors cannot detect inorganic substances and some highly oxygenated or functionalized species like infrared and laser technology can. In some systems, CO and CO2 can be detected in the FID using amethanizer
Methanizer is an appliance used in gas chromatography (GC), which allows the user to detect very low concentrations of carbon monoxide and carbon dioxide. It consists of a flame ionization detector, preceded by a hydrogenating reactor, which conve ...
, which is a bed of Ni catalyst that reduces CO and CO2 to methane, which can be in turn detected by the FID. The methanizer
Methanizer is an appliance used in gas chromatography (GC), which allows the user to detect very low concentrations of carbon monoxide and carbon dioxide. It consists of a flame ionization detector, preceded by a hydrogenating reactor, which conve ...
is limited by its inability to reduce compounds other than CO and CO2 and its tendency to be poisoned by a number of chemicals commonly found in gas chromatography effluents.
Another important disadvantage is that the FID flame oxidizes all oxidizable compounds that pass through it; all hydrocarbons and oxygenates are oxidized to carbon dioxide and water and other heteroatoms are oxidized according to thermodynamics. For this reason, FIDs tend to be the last in a detector train and also cannot be used for preparatory work.
Alternative solution
An improvement to themethanizer
Methanizer is an appliance used in gas chromatography (GC), which allows the user to detect very low concentrations of carbon monoxide and carbon dioxide. It consists of a flame ionization detector, preceded by a hydrogenating reactor, which conve ...
is the Polyarc reactor
The Polyarc reactor is a scientific tool for the measurement of organic molecules. It is paired with a flame ionization detector (FID) in a gas chromatograph (GC) to improve the sensitivity of the FID and give a uniform detector response for all o ...
, which is a sequential reactor that oxidizes compounds before reducing them to methane. This method can be used to improve the response of the FID and allow for the detection of many more carbon-containing compounds. The complete conversion of compounds to methane and the now equivalent response in the detector also eliminates the need for calibrations and standards because response factors are all equivalent to those of methane. This allows for the rapid analysis of complex mixtures that contain molecules where standards are not available.
See also
*Flame detector
A flame detector is a sensor designed to detect and respond to the presence of a flame or fire, allowing flame detection. Responses to a detected flame depend on the installation, but can include sounding an alarm, deactivating a fuel line (such a ...
*Thermal conductivity detector The thermal conductivity detector (TCD), also known as a katharometer, is a bulk property detector and a chemical specific detector commonly used in gas chromatography. This detector senses changes in the thermal conductivity of the column eluent an ...
*Gas chromatography
Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, ...
*Active fire protection
Active fire protection (AFP) is an integral part of fire protection. AFP is characterized by items and/or systems, which require a certain amount of motion and response in order to work, contrary to passive fire protection.
Categories of active ...
* Photoionization detector
*Photoelectric flame photometer
A photoelectric flame photometer is an instrument used in inorganic chemical analysis to determine the concentration of certain metal ions, among them sodium, potassium, lithium, and calcium. Group 1 and Group 2 metals are quite sensitive to Flame ...
References
Sources
*Skoog, Douglas A., F. James Holler, & Stanley R. Crouch. Principles of Instrumental Analysis. 6th Edition. United States: Thomson Brooks/Cole, 2007. * *G.H. JEFFERY, J.BASSET, J.MENDHAM, R.C.DENNEY, "VOGEL'S TEXTBOOK OF QUANTITATIVE CHEMICAL ANALYSIS." {{refend Gas chromatography Australian inventions South African inventions