FlAsH-EDT2 on:
[Wikipedia]
[Google]
[Amazon]
FlAsH-EDT2 is an
 FlAsH-EDT2 can be prepared in three steps from
FlAsH-EDT2 can be prepared in three steps from
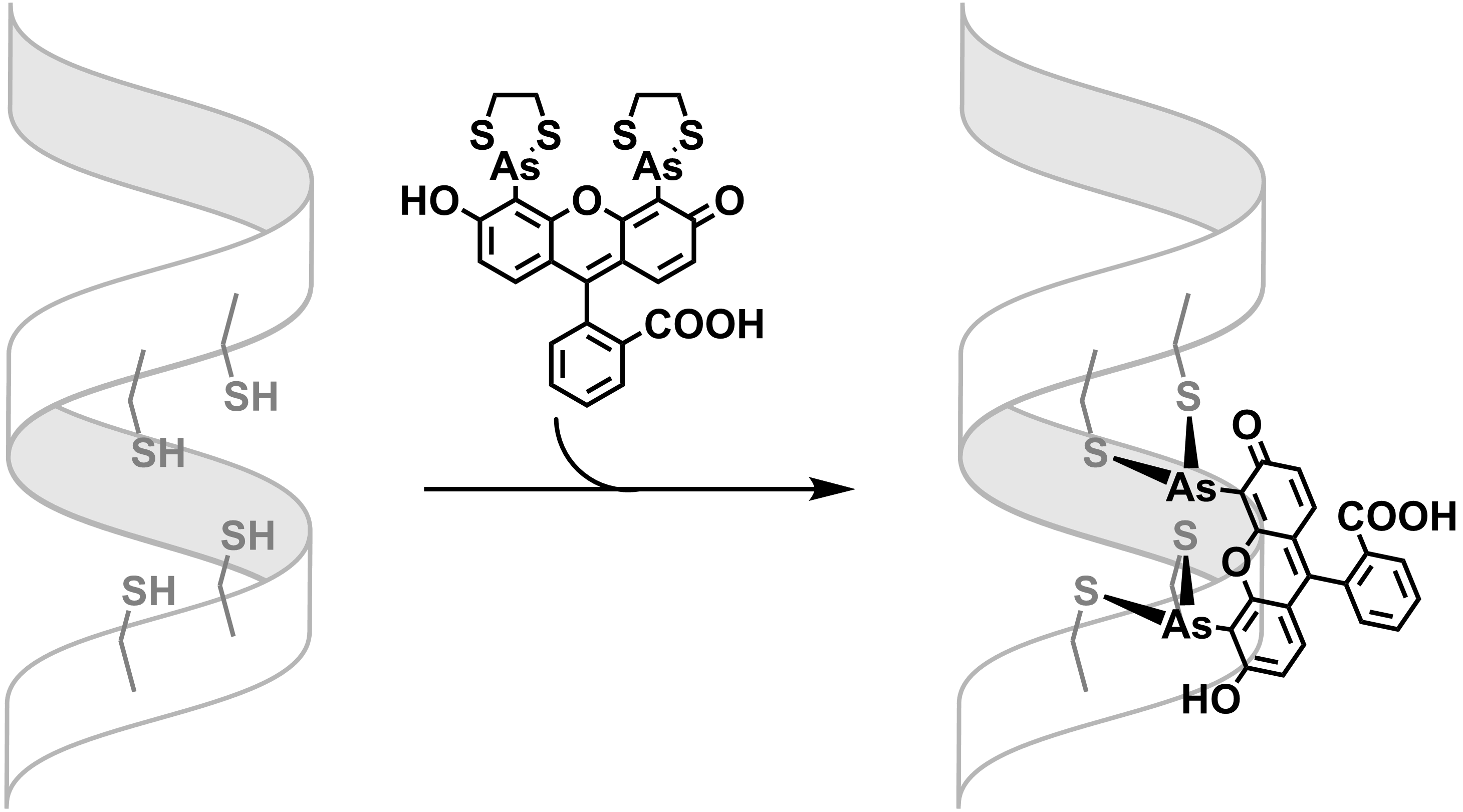 The binding of FlAsH-EDT2 is thus subject to equilibration. The FlAsH-peptide adduct formation can be favored in low concentration of EDT (below 10
The binding of FlAsH-EDT2 is thus subject to equilibration. The FlAsH-peptide adduct formation can be favored in low concentration of EDT (below 10
/ref> It has been proven to be a good alternative to green fluorescent proteins (GFP) with the advantage that FlAsH-EDT2 is much smaller (
organoarsenic compound
Organoarsenic chemistry is the chemistry of Chemical compound, compounds containing a chemical bond between arsenic and carbon. A few organoarsenic compounds, also called "organoarsenicals," are produced industrially with uses as insecticides, herb ...
with molecular formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as paren ...
C24H18As2O5S4. Its structure is based around a fluorescein
Fluorescein is an organic compound and dye based on the xanthene tricyclic structural motif, formally belonging to Triarylmethane dye, triarylmethine dyes family. It is available as a dark orange/red powder slightly soluble in water and alcohol. ...
core with two 1,3,2-dithiarsolane substituents. It is used in bioanalytical research as a fluorescent label for visualising protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
s in living cells. FlAsH-EDT2 is an abbreviation for fluorescin arsenical hairpin binder- ethanedithiol, and is a pale yellow or pinkish fluorogenic solid. It has a semi-structural formula
The structural formula of a chemical compound is a graphic representation of the molecular structure (determined by structural chemistry methods), showing how the atoms are connected to one another. The chemical bonding within the molecule is al ...
(C2H4AsS2)2-(C13H5O3)-C6H4COOH, representing the dithiarsolane substituents bound to the hydroxyxanthone
Xanthone is an organic compound with the molecular formula C13H8O2. It is a white solid.
In 1939, xanthone was introduced as an insecticide and it currently finds uses as ovicide for codling moth eggs and as a larvicide. Xanthone is also use ...
core, attached to an ''o''-substituted molecule of benzoic acid
Benzoic acid () is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. The benzoyl group is often abbreviated "Bz" (not to be confused with "Bn," which ...
.
FlAsH-EDT2 is used for site-specific labelling, selectively binding to proteins containing the tetracysteine
Cysteine (; symbol Cys or C) is a semiessential proteinogenic amino acid with the chemical formula, formula . The thiol side chain in cysteine enables the formation of Disulfide, disulfide bonds, and often participates in enzymatic reactions as ...
(TC) motif Cys-Cys-Xxx-Xxx-Cys-Cys and becoming fluorescent when bound. It displays non-specific binding to endogenous cysteine-rich proteins, meaning it binds to sites other than the one of interest (CCXXCC). Further optimization of the TC motif has revealed improved FlAsH binding affinity for a CCPGCC motif, and higher quantum yield
In particle physics, the quantum yield (denoted ) of a radiation-induced process is the number of times a specific event occurs per photon absorbed by the system.
\Phi(\lambda)=\frac
Applications
Fluorescence spectroscopy
The fluorescence ...
when the tetracysteine motif is flanked with specific residues (HRWCCPGCCKTF or FLNCCPGCCMEP).
Preparation
 FlAsH-EDT2 can be prepared in three steps from
FlAsH-EDT2 can be prepared in three steps from fluorescein
Fluorescein is an organic compound and dye based on the xanthene tricyclic structural motif, formally belonging to Triarylmethane dye, triarylmethine dyes family. It is available as a dark orange/red powder slightly soluble in water and alcohol. ...
(see figure).
Formation of FlAsH-TC adduct
: Many studies show that trivalent arsenic compounds bind to pairs of cysteine residues. This binding is responsible for the toxicity of many arsenic compounds. Binding is reversed by 1,2-ethanedithiol, which binds tightly to arsenic compounds, as shown by the stability of FlAsH-EDT2. Such strong sulfur-arsenic bond can be, again, regulated by designing a peptide domain that exhibits higher affinity toward the arsenic, such as tetracysteine motif. By modulating the distance between the two pairs of cysteine residues and the space between the arsenic centers of FlAsH-EDT2, a cooperative and entropically favored dithiol arsenic bond could be achieved. The binding of FlAsH-EDT2 is thus subject to equilibration. The FlAsH-peptide adduct formation can be favored in low concentration of EDT (below 10
The binding of FlAsH-EDT2 is thus subject to equilibration. The FlAsH-peptide adduct formation can be favored in low concentration of EDT (below 10 μM
The micrometre (Commonwealth English as used by the International Bureau of Weights and Measures; SI symbol: μm) or micrometer (American English), also commonly known by the non-SI term micron, is a unit of length in the International System ...
) and be reversed in high concentration of EDT (above 1 mM).
Properties
FlAsH becomes fluorescent upon the binding of tetracysteine motif. It is excited at 508 nm and emits 528 nm, a green-yellow, of free fluorescein. Thequantum yield
In particle physics, the quantum yield (denoted ) of a radiation-induced process is the number of times a specific event occurs per photon absorbed by the system.
\Phi(\lambda)=\frac
Applications
Fluorescence spectroscopy
The fluorescence ...
is 0.49 for 250 nM FlAsH is bound to a model tetracysteine-containing peptide in a phosphate-buffered saline at pH 7.4.
Generally, FlAsH-EDT2 has 0.1-0.6 fluorescence quantum efficiencies with several μM detection limits for diffuse cytosolic tag and 30 - 80 extinction coefficients L mmol−1 cm−1. The FlAsH-peptide complex also has demonstrated fluorescence resonance energy transfer
Fluorescence is one of two kinds of photoluminescence, the emission of light by a substance that has absorbed light or other electromagnetic radiation. When exposed to ultraviolet radiation, many substances will glow (fluoresce) with colore ...
(FRET) from fluorescent proteins, such as from enhanced cyan fluorescent protein (ECFP) of Green Fluorescent Protein
The green fluorescent protein (GFP) is a protein that exhibits green fluorescence when exposed to light in the blue to ultraviolet range. The label ''GFP'' traditionally refers to the protein first isolated from the jellyfish ''Aequorea victo ...
(GFP).
Application
FlAsH-EDT2 enables less toxic and more specific fluorescent labeling that is membrane permeable. The modification of the fluorescein moiety also allows multicolor analysis.TC-FlAsH™ II In-Cell Tetracysteine Tag Detection Kit (Green Fluorescence), for live-cell imaging/ref> It has been proven to be a good alternative to green fluorescent proteins (GFP) with the advantage that FlAsH-EDT2 is much smaller (
molar mass
In chemistry, the molar mass () (sometimes called molecular weight or formula weight, but see related quantities for usage) of a chemical substance ( element or compound) is defined as the ratio between the mass () and the amount of substance ...
< 1 kDa
The dalton or unified atomic mass unit (symbols: Da or u, respectively) is a unit of mass defined as of the mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state and at rest. It is a non-SI unit accepted f ...
) as compared to GFPs (~30 kDa), therefore minimizing the perturbation of activity of the protein under the study.
Use
In the past, FlAsH-EDT2 has been widely used to study a number of ''in vivo'' cellular events and subcellular structures in animal cells, Ebola virus matrix protein, and protein misfolding. With the electron microscopic imaging, FlAsH-EDT2 is also used to study the processes of protein trafficking ''in situ''. More recently, it was used in an extended study of plant cells like ''Arabidopsis
''Arabidopsis'' (rockcress) is a genus in the family Brassicaceae. They are small flowering plants related to cabbage and mustard. This genus is of great interest since it contains thale cress (''Arabidopsis thaliana''), one of the model organ ...
'' and tobacco.
References
{{Reflist Biochemistry detection reactions Fluorescent dyes Hydroxyarenes Benzoic acids Triarylmethane dyes Organoarsenic dithiolates Arsenic heterocycles