Ferrocene on:
[Wikipedia]
[Google]
[Amazon]
Ferrocene is an organometallic compound with the formula . The molecule is a
 Pauson and Kealy conjectured that the compound had two cyclopentadienyl groups, each with a single covalent bond from the saturated carbon atom to the iron atom. However, that structure was inconsistent with then-existing bonding models and did not explain the unexpected stability of the compound, and chemists struggled to find the correct structure.
The structure was deduced and reported independently by three groups in 1952. Robert Burns Woodward, Geoffrey Wilkinson, et al. observed that the compound was diamagnetic and nonpolar. A few months later they described its reactions as being typical of aromatic compounds such as
Pauson and Kealy conjectured that the compound had two cyclopentadienyl groups, each with a single covalent bond from the saturated carbon atom to the iron atom. However, that structure was inconsistent with then-existing bonding models and did not explain the unexpected stability of the compound, and chemists struggled to find the correct structure.
The structure was deduced and reported independently by three groups in 1952. Robert Burns Woodward, Geoffrey Wilkinson, et al. observed that the compound was diamagnetic and nonpolar. A few months later they described its reactions as being typical of aromatic compounds such as

 Another early synthesis of ferrocene was by Miller ''et al.'', who treated metallic iron with gaseous cyclopentadiene at elevated temperature. An approach using iron pentacarbonyl was also reported.
:Fe(CO)5 + 2 C5H6 → Fe(C5H5)2 + 5 CO + H2
Another early synthesis of ferrocene was by Miller ''et al.'', who treated metallic iron with gaseous cyclopentadiene at elevated temperature. An approach using iron pentacarbonyl was also reported.
:Fe(CO)5 + 2 C5H6 → Fe(C5H5)2 + 5 CO + H2
 Ferrocene is an
Ferrocene is an
 Ferrocene undergoes a one-electron oxidation at around 0.4 V versus a saturated calomel electrode (SCE), becoming ferrocenium. This reversible oxidation has been used as standard in electrochemistry as Fc+/Fc = 0.64 V versus the standard hydrogen electrode, however other values have been reported. Ferrocenium tetrafluoroborate is a common reagent. The remarkably reversible oxidation-reduction behaviour has been extensively used to control electron-transfer processes in electrochemical and photochemical systems.
Substituents on the cyclopentadienyl ligands alters the redox potential in the expected way: electron-withdrawing groups such as a
Ferrocene undergoes a one-electron oxidation at around 0.4 V versus a saturated calomel electrode (SCE), becoming ferrocenium. This reversible oxidation has been used as standard in electrochemistry as Fc+/Fc = 0.64 V versus the standard hydrogen electrode, however other values have been reported. Ferrocenium tetrafluoroborate is a common reagent. The remarkably reversible oxidation-reduction behaviour has been extensively used to control electron-transfer processes in electrochemical and photochemical systems.
Substituents on the cyclopentadienyl ligands alters the redox potential in the expected way: electron-withdrawing groups such as a
 Ferrocene derivatives have been investigated as drugs, with one compound approved for use in the USSR in the 1970s as an iron supplement, though it is no longer marketed today. Only one drug has entered clinical trials in recent years, Ferroquine (7-chloro-N-(2-((dimethylamino)methyl)ferrocenyl)quinolin-4-amine), an antimalarial, which has reached Phase IIb trials. Ferrocene-containing polymer-based drug delivery systems have been investigated.
The anticancer activity of ferrocene derivatives was first investigated in the late 1970s, when derivatives bearing
Ferrocene derivatives have been investigated as drugs, with one compound approved for use in the USSR in the 1970s as an iron supplement, though it is no longer marketed today. Only one drug has entered clinical trials in recent years, Ferroquine (7-chloro-N-(2-((dimethylamino)methyl)ferrocenyl)quinolin-4-amine), an antimalarial, which has reached Phase IIb trials. Ferrocene-containing polymer-based drug delivery systems have been investigated.
The anticancer activity of ferrocene derivatives was first investigated in the late 1970s, when derivatives bearing
 Ferrocene, a precursor to iron nanoparticles, can be used as a catalyst for the production of carbon nanotubes. Vinylferrocene can be converted to (polyvinylferrocene, PVFc), a ferrocenyl version of
Ferrocene, a precursor to iron nanoparticles, can be used as a catalyst for the production of carbon nanotubes. Vinylferrocene can be converted to (polyvinylferrocene, PVFc), a ferrocenyl version of
Ferrocene
at ''
NIOSH Pocket Guide to Chemical Hazards
(Centers for Disease Control and Prevention) {{Authority control Antiknock agents Sandwich compounds Cyclopentadienyl complexes Substances discovered in the 1950s
complex
Complex commonly refers to:
* Complexity, the behaviour of a system whose components interact in multiple ways so possible interactions are difficult to describe
** Complex system, a system composed of many components which may interact with each ...
consisting of two cyclopentadienyl rings sandwiching a central iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
atom. It is an orange solid with a camphor-like odor that sublimes above room temperature, and is soluble in most organic solvents. It is remarkable for its stability: it is unaffected by air, water, strong bases, and can be heated to 400 °C without decomposition. In oxidizing conditions it can reversibly react with strong acids to form the ferrocenium cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
. Ferrocene and the ferrocenium cation are sometimes abbreviated as Fc and respectively.
The first reported synthesis of ferrocene was in 1951. Its unusual stability puzzled chemists, and required the development of new theory to explain its formation and bonding. The discovery of ferrocene and its many analogues, known as metallocenes, sparked excitement and led to a rapid growth in the discipline of organometallic chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
. Geoffrey Wilkinson and Ernst Otto Fischer, both of whom worked on elucidating the structure of ferrocene, later shared the 1973 Nobel Prize in Chemistry for their work on organometallic sandwich compounds. Ferrocene itself has no large-scale applications, but has found more niche uses in catalysis, as a fuel additive, and as a tool in undergraduate education.
History
Discovery
Ferrocene was discovered by accident twice. The first known synthesis may have been made in the late 1940s by unknown researchers at Union Carbide, who tried to pass hot cyclopentadiene vapor through an iron pipe. The vapor reacted with the pipe wall, creating a "yellow sludge" that clogged the pipe. Years later, a sample of the sludge that had been saved was obtained and analyzed by Eugene O. Brimm, shortly after reading Kealy and Pauson's article, and was found to consist of ferrocene. The second time was around 1950, when Samuel A. Miller, John A. Tebboth, and John F. Tremaine, researchers at British Oxygen, were attempting to synthesize amines from hydrocarbons andnitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
in a modification of the Haber process
The Haber process, also called the Haber–Bosch process, is the main industrial procedure for the ammonia production, production of ammonia. It converts atmospheric nitrogen (N2) to ammonia (NH3) by a reaction with hydrogen (H2) using finely di ...
. When they tried to react cyclopentadiene with nitrogen at 300 °C, at atmospheric pressure, they were disappointed to see the hydrocarbon react with some source of iron, yielding ferrocene. While they too observed its remarkable stability, they put the observation aside and did not publish it until after Pauson reported his findings. Kealy and Pauson were later provided with a sample by Miller ''et al.'', who confirmed that the products were the same compound.
In 1951, Peter L. Pauson and Thomas J. Kealy at Duquesne University attempted to prepare fulvalene () by oxidative dimerization of cyclopentadiene (). To that end, they reacted the Grignard compound cyclopentadienyl magnesium bromide in diethyl ether
Diethyl ether, or simply ether, is an organic compound with the chemical formula , sometimes abbreviated as . It is a colourless, highly Volatility (chemistry), volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It belongs ...
with ferric chloride
Iron(III) chloride describes the inorganic compounds with the formula (H2O)x. Also called ferric chloride, these compounds are some of the most important and commonplace compounds of iron. They are available both in anhydrous and in hydrated f ...
as an oxidizer. However, instead of the expected fulvalene, they obtained a light orange powder of "remarkable stability", with the formula .
Determining the structure
benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
. The name ferrocene was coined by Mark Whiting, a postdoc with Woodward. Ernst Otto Fischer and Wolfgang Pfab also noted ferrocene's diamagneticity and high symmetry. They also synthesized nickelocene and cobaltocene and confirmed they had the same structure. Fischer described the structure as ''Doppelkegelstruktur'' ("double-cone structure"), although the term "sandwich" came to be preferred by British and American chemists. Philip Frank Eiland and Raymond Pepinsky confirmed the structure through X-ray crystallography
X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to Diffraction, diffract in specific directions. By measuring th ...
and later by NMR
Nuclear magnetic resonance (NMR) is a physical phenomenon in which atomic nucleus, nuclei in a strong constant magnetic field are disturbed by a weak oscillating magnetic field (in the near and far field, near field) and respond by producing ...
spectroscopy.
The "sandwich" structure of ferrocene was shockingly novel and led to intensive theoretical studies. Application of molecular orbital theory with the assumption of a Fe2+ centre between two cyclopentadienide anions resulted in the successful Dewar–Chatt–Duncanson model, allowing correct prediction of the geometry of the molecule as well as explaining its remarkable stability.
Impact
The discovery of ferrocene was considered so significant that Wilkinson and Fischer shared the 1973 Nobel Prize in Chemistry "for their pioneering work, performed independently, on the chemistry of the organometallic, called sandwich compounds".Structure and bonding
Mössbauer spectroscopy indicates that the iron center in ferrocene should be assigned the +2 oxidation state. Each cyclopentadienyl (Cp) ring should then be allocated a single negative charge. Thus ferrocene could be described as iron(II) bis( cyclopentadienide), . Each ring has six π-electrons, which makes themaromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugati ...
according to Hückel's rule. These π-electrons are then shared with the metal via covalent bonding. Since Fe2+ has six ''d''-electrons, the complex attains an 18-electron configuration, which accounts for its stability. In modern notation, this sandwich structural model of the ferrocene molecule is denoted as , where ''η'' denotes hapticity, the number of atoms through which each ring binds.
The carbon–carbon bond distances around each five-membered ring are all 1.40 Å, and all Fe–C bond distances are 2.04 Å. The Cp rings rotate with a low barrier about the Cp(centroid)–Fe–Cp(centroid) axis, as observed by measurements on substituted derivatives of ferrocene using 1H and 13C nuclear magnetic resonance
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are disturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with a ...
spectroscopy. For example, methylferrocene (CH3C5H4FeC5H5) exhibits a singlet for the C5H5 ring.
From room temperature down to 164 K, X-ray crystallography
X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to Diffraction, diffract in specific directions. By measuring th ...
yields the monoclinic space group; the cyclopentadienide rings are a staggered conformation, resulting in a centrosymmetric molecule, with symmetry group
In group theory, the symmetry group of a geometric object is the group of all transformations under which the object is invariant, endowed with the group operation of composition. Such a transformation is an invertible mapping of the amb ...
D5d. However, below 110 K, ferrocene crystallizes in an orthorhombic crystal lattice in which the Cp rings are ordered and eclipsed, so that the molecule has symmetry group D5h. In the gas phase, electron diffraction and computational studies show that the Cp rings are eclipsed. While ferrocene has no permanent dipole moment at room temperature, between 172.8 and 163.5 K the molecule exhibits an "incommensurate modulation", breaking the D5 symmetry and acquiring an electric dipole.
In solution, eclipsed D5h ferrocene was determined to dominate over the staggered D5d conformer, as suggested by both Fourier-transform infrared spectroscopy and DFT calculations.
Synthesis
Early methods
The first reported syntheses of ferrocene were nearly simultaneous. Pauson and Kealy synthesised ferrocene using iron(III) chloride and cyclopentadienyl magnesium bromide. Aredox reaction
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
produces iron(II) chloride. The formation of fulvalene (the intended outcome), does not occur.
:
Via alkali cyclopentadienide
More efficient preparative methods are generally a modification of the original transmetalation sequence using either commercially availablesodium cyclopentadienide
Sodium cyclopentadienide is an organosodium compound with the formula C5H5Na. The compound is often abbreviated as NaCp, where Cp− is the cyclopentadienide anion. Sodium cyclopentadienide is a colorless solid, although samples often are pin ...
or freshly cracked cyclopentadiene deprotonated with potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash.
Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which utili ...
and reacted with anhydrous iron(II) chloride in ethereal solvents.
Modern modifications of Pauson and Kealy's original Grignard approach are known:
*Using sodium cyclopentadienide: 2 NaC5H5 + FeCl2 → Fe(C5H5)2 + 2 NaCl
*Using freshly-cracked cyclopentadiene: FeCl2·4H2O + 2 C5H6 + 2 KOH → Fe(C5H5)2 + 2 KCl + 6 H2O
*Using an iron(II) salt with a Grignard reagent: 2 C5H5MgBr + FeCl2 → Fe(C5H5)2 + 2 MgBrCl
Even some amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
bases (such as diethylamine) can be used for the deprotonation, though the reaction proceeds more slowly than when using stronger bases:
:2 C5H6 + 2 (CH3CH2)2NH + FeCl2 → Fe(C5H5)2 + 2 (CH3CH2)2NH2Cl
Direct transmetalation can also be used to prepare ferrocene from some other metallocenes, such as manganocene:
:FeCl2 + Mn(C5H5)2 → MnCl2 + Fe(C5H5)2
Properties
 Ferrocene is an
Ferrocene is an air
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosph ...
-stable orange solid with a camphor-like odor. As expected for a symmetric, uncharged species, ferrocene is soluble in normal organic solvents, such as benzene, but is insoluble in water. It is stable to temperatures as high as 400 °C.
Ferrocene readily sublimes, especially upon heating in a vacuum. Its vapor pressure is about 1 Pa at 25 °C, 10 Pa at 50 °C, 100 Pa at 80 °C, 1000 Pa at 116 °C, and 10,000 Pa (nearly 0.1 atm) at 162 °C.
Reactions
Aromatic substitution
Ferrocene is an aromatic substance. Electrophiles typically substitute onto, rather than add to, the cyclopentadienyl ligands. For example, a common undergraduate experiment performs Friedel-Crafts acylation withacetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the chemical formula, formula . Commonly abbreviated , it is one the simplest organic acid anhydride, anhydrides of a carboxylic acid and is widely used in the production of c ...
and a phosphoric acid
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula . It is commonly encountered as an 85% aqueous solution, ...
catalyst. Just as this reagent mixture converts benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
to acetophenone, it converts ferrocene to acetylferrocene.
In the presence of aluminium chloride
Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula . It forms a hexahydrate with the formula , containing six water molecules of hydration. Both the anhydrous form and the hexahydrate are col ...
, Me2NPCl2 and ferrocene react to give ferrocenyl dichlorophosphine, whereas treatment with phenyldichlorophosphine under similar conditions forms ''P'',''P''-diferrocenyl-''P''-phenyl phosphine. Vilsmeier-Haack formylation using formylanilide and phosphorus oxychloride gives ferrocenecarboxaldehyde.
Unsubstituted ferrocene undergoes aromatic substitution more easily than benzene, because electrophiles can attack the metal ion before rearranging to the Wheland intermediate. Thus ferrocene reacts with the weak electrophile P4S10 to form a diferrocenyl-dithiadiphosphetane disulfide. Mannich conditions suffice to iminylate ferrocene unto N,N-dimethylaminomethylferrocene.
Superacidic protonation does not complete aromatic substitution, but rather traps the unrearranged bent intermediate hydrido salt, p2FeHF6. Strongly oxidizing electrophiles, such as halogens and nitric acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most com ...
, neither rearrange to a Wheland intermediate nor coordinate to iron, instead generating ferrocenium salts (see ).
In accordance with cluster compound theory, ferrocene's rings behave as a single delocalized π system. Electronic perturbations to one ring propagate to the other. For example, introduction of a deactivating aldehyde group on one ring inhibits formylation of the other ring as well.
Metallation
Ferrocene readily metallates. Ferrocene reacts with butyllithium to give 1,1-dilithioferrocene, which is a versatilenucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
. In combination with butyllithiium, ''tert''-butyllithium produces monolithioferrocene. Likewise ferrocene mercurates to give ferrocendiyl dimercuriacetate.
Further reaction gives the nitro
Nitro may refer to:
Chemistry
*Nitrogen, a chemical element and a gas except at very low temperatures, with which many compounds are formed:
**Nitro compound, an organic compound containing one or more nitro functional groups, -NO2
**Nitro ligand ...
, halo-, and borono derivatives.
Redox chemistry
carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
shift the potential in the anodic direction (''i.e.'' made more positive), whereas electron-releasing groups such as methyl groups shift the potential in the cathodic
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional current ...
direction (more negative). Thus, decamethylferrocene is much more easily oxidised than ferrocene and can even be oxidised to the corresponding dication. Ferrocene is often used as an internal standard for calibrating redox potentials in non-aqueous electrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between Electric potential, electrical potential difference and identifiable chemical change. These reactions involve Electron, electrons moving via an electronic ...
.
Stereochemistry of substituted ferrocenes
Disubstituted ferrocenes can exist as either 1,2-, 1,3- or 1,1- isomers, none of which are interconvertible. Ferrocenes that are asymmetrically disubstituted on one ring are chiral – for example pFe(EtC5H3Me) This planar chirality arises despite no single atom being a stereogenic centre. Professor Fu's planar DMAP has been demonstrated as an affective catalyst for various asymmetric transformations including thekinetic resolution
In organic chemistry, kinetic resolution is a means of differentiating two enantiomers in a racemic mixture. In kinetic resolution, two enantiomers react with different reaction rates in a chemical reaction with a chiral catalyst or reagent, re ...
of racemic secondary alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
s.
Several approaches have been developed to asymmetrically 1,1-functionalise the ferrocene.
Applications of ferrocene and its derivatives
Ferrocene and its numerous derivatives have no large-scale applications, but have many niche uses that exploit the unusual structure (ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
scaffolds, pharmaceutical candidates), robustness
Robustness is the property of being strong and healthy in constitution. When it is transposed into a system
A system is a group of interacting or interrelated elements that act according to a set of rules to form a unified whole. A system, ...
( anti-knock formulations, precursors to materials), and redox (reagents and redox standards).
Ligand scaffolds
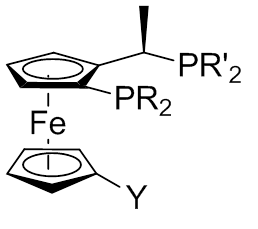
Chiral ferrocenylphosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
s are employed as ligands for transition-metal catalyzed reactions. Some of them have found industrial applications in the synthesis of pharmaceuticals and agrochemicals. For example, the diphosphine 1,1-bis(diphenylphosphino)ferrocene (dppf) is a valued ligand for palladium
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), ...
- coupling reactions and Josiphos ligand is useful for hydrogenation catalysis. They are named after the technician who made the first one, Josi Puleo.

Fuel additives
Ferrocene and its derivatives are antiknock agents used in the fuel forpetrol engine
A petrol engine (gasoline engine in American and Canadian English) is an internal combustion engine designed to run on petrol (gasoline). Petrol engines can often be adapted to also run on fuels such as liquefied petroleum gas and ethanol blends ...
s. They are safer than previously used tetraethyllead
Tetraethyllead (commonly styled tetraethyl lead), abbreviated TEL, is an organolead compound with the formula lead, Pb(ethyl group, C2H5)4. It was widely used as a fuel additive for much of the 20th century, first being mixed with gasoline begi ...
. Petrol additive solutions containing ferrocene can be added to unleaded petrol to enable its use in vintage cars designed to run on leaded petrol. The iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
-containing deposits formed from ferrocene can form a conductive
In physics and electrical engineering, a conductor is an object or type of material that allows the flow of Electric charge, charge (electric current) in one or more directions. Materials made of metal are common electrical conductors. The flow ...
coating on spark plug surfaces. Ferrocene polyglycol copolymers, prepared by effecting a polycondensation reaction between a ferrocene derivative and a substituted dihydroxy alcohol, has promise as a component of rocket propellants. These copolymers provide rocket propellants with heat stability, serving as a propellant binder and controlling propellant burn rate.
Ferrocene has been found to be effective at reducing smoke and sulfur trioxide produced when burning coal. The addition by any practical means, impregnating the coal or adding ferrocene to the combustion chamber, can significantly reduce the amount of these undesirable byproducts, even with a small amount of the metal cyclopentadienyl compound.
Pharmaceuticals
amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
or amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a chemical compound, compound with the general formula , where R, R', and R″ represent any group, typically organyl functional group, groups or hydrogen at ...
groups were tested against lymphocytic leukemia
Leukemia ( also spelled leukaemia; pronounced ) is a group of blood cancers that usually begin in the bone marrow and produce high numbers of abnormal blood cells. These blood cells are not fully developed and are called ''blasts'' or '' ...
. Some ferrocenium salts exhibit anticancer activity, but no compound has seen evaluation in the clinic. Ferrocene derivatives have strong inhibitory activity against human lung cancer cell line A549, colorectal cancer cell line HCT116, and breast cancer cell line MCF-7. An experimental drug was reported which is a ferrocenyl version of tamoxifen. The idea is that the tamoxifen will bind to the estrogen
Estrogen (also spelled oestrogen in British English; see spelling differences) is a category of sex hormone responsible for the development and regulation of the female reproductive system and secondary sex characteristics. There are three ...
binding sites, resulting in cytotoxicity.
Ferrocifens are exploited for cancer applications by a French biotech, Feroscan, founded by Pr. Gerard Jaouen.
Solid rocket propellant
Ferrocene and related derivatives are used as powerful burn rate catalysts in ammonium perchlorate composite propellant.Derivatives and variations
Ferrocene analogues can be prepared with variants of cyclopentadienyl. For example, bis indenyliron and bisfluorenyliron. Carbon atoms can be replaced by heteroatoms as illustrated by Fe(''η''5-C5Me5)(''η''5-P5) and Fe(''η''5-C5H5)(''η''5-C4H4N) (" azaferrocene"). Azaferrocene arises from decarbonylation of Fe(''η''5-C5H5)(CO)2(''η''1-pyrrole) incyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colourless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexan ...
. This compound on boiling under reflux in benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
is converted to ferrocene.
The bis(benzene)iron(II) cation, isoelectronic with bis(benzene)chromium, is unstable against nucleophilic attack, and decomposes "instantaneously" in acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not class ...
. It can be observed, however, in metastable nitromethane solution.
Because of the ease of substitution, many structurally unusual ferrocene derivatives have been prepared. For example, the penta(ferrocenyl)cyclopentadienyl ligand, features a cyclopentadienyl anion derivatized with five ferrocene substituents.
In hexaferrocenylbenzene, C6 ''η''5-C5H4)Fe(''η''5-C5H5)sub>6, all six positions on a benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
molecule have ferrocenyl substituents (R). X-ray diffraction
X-ray diffraction is a generic term for phenomena associated with changes in the direction of X-ray beams due to interactions with the electrons around atoms. It occurs due to elastic scattering, when there is no change in the energy of the waves. ...
analysis of this compound confirms that the cyclopentadienyl ligands are not co-planar with the benzene core but have alternating dihedral angles of +30° and −80°. Due to steric crowding the ferrocenyls are slightly bent with angles of 177° and have elongated C-Fe bonds. The quaternary cyclopentadienyl carbon atoms are also pyramidalized. Also, the benzene core has a chair conformation with dihedral angles of 14° and displays bond length
In molecular geometry, bond length or bond distance is defined as the average distance between Atomic nucleus, nuclei of two chemical bond, bonded atoms in a molecule. It is a Transferability (chemistry), transferable property of a bond between at ...
alternation between 142.7 pm and 141.1 pm, both indications of steric crowding of the substituents.
The synthesis of hexaferrocenylbenzene has been reported using Negishi coupling of hexaiodobenzene and diferrocenylzinc, using tris(dibenzylideneacetone)dipalladium(0) as catalyst, in tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
:
:
The yield is only 4%, which is further evidence consistent with substantial steric crowding around the arene core.
Materials chemistry
 Ferrocene, a precursor to iron nanoparticles, can be used as a catalyst for the production of carbon nanotubes. Vinylferrocene can be converted to (polyvinylferrocene, PVFc), a ferrocenyl version of
Ferrocene, a precursor to iron nanoparticles, can be used as a catalyst for the production of carbon nanotubes. Vinylferrocene can be converted to (polyvinylferrocene, PVFc), a ferrocenyl version of polystyrene
Polystyrene (PS) is a synthetic polymer made from monomers of the aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It i ...
(the phenyl groups are replaced with ferrocenyl groups). Another polyferrocene which can be formed is poly(2-(methacryloyloxy)ethyl ferrocenecarboxylate), PFcMA. In addition to using organic polymer backbones, these pendant ferrocene units have been attached to inorganic backbones such as polysiloxanes, polyphosphazenes, and poly phosphinoboranes, (–PH(R)–BH2–)''n'', and the resulting materials exhibit unusual physical and electronic properties relating to the ferrocene / ferrocinium redox couple. Both PVFc and PFcMA have been tethered onto silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant f ...
wafers and the wettability measured when the polymer chains are uncharged and when the ferrocene moieties are oxidised to produce positively charged groups. The contact angle with water on the PFcMA-coated wafers was 70° smaller following oxidation, while in the case of PVFc the decrease was 30°, and the switching of wettability is reversible. In the PFcMA case, the effect of lengthening the chains and hence introducing more ferrocene groups is significantly larger reductions in the contact angle upon oxidation.
See also
* Josiphos ligandsReferences
External links
Ferrocene
at ''
The Periodic Table of Videos
''Periodic Videos'' (also known as ''The Periodic Table of Videos'') is a video project and YouTube channel on chemistry. It consists of a series of videos about chemical elements and the periodic table, with additional videos on other topics i ...
'' (University of Nottingham)NIOSH Pocket Guide to Chemical Hazards
(Centers for Disease Control and Prevention) {{Authority control Antiknock agents Sandwich compounds Cyclopentadienyl complexes Substances discovered in the 1950s