Ferredoxin-thioredoxin Reductase on:
[Wikipedia]
[Google]
[Amazon]
Ferredoxin-thioredoxin reductase , systematic name ''ferredoxin:thioredoxin disulfide oxidoreductase,'' is a Fe-4S
 The variable α subunit has an open β barrel structure made of five antiparallel β strands. Its interaction with the catalytic subunit occurs mainly with two loops between β strands. The residues in these two loops are mostly conserved and are thought to stabilize the 4Fe-4S cluster in the catalytic subunit. Structurally, the α subunit is very similar to the PsaE protein, a subunit of
The variable α subunit has an open β barrel structure made of five antiparallel β strands. Its interaction with the catalytic subunit occurs mainly with two loops between β strands. The residues in these two loops are mostly conserved and are thought to stabilize the 4Fe-4S cluster in the catalytic subunit. Structurally, the α subunit is very similar to the PsaE protein, a subunit of
protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
that plays an important role in the ferredoxin
Ferredoxins (from Latin ''ferrum'': iron + redox, often abbreviated "fd") are iron–sulfur proteins that mediate electron transfer in a range of metabolic reactions. The term "ferredoxin" was coined by D.C. Wharton of the DuPont Co. and applied t ...
/thioredoxin
Thioredoxin (TRX or TXN) is a class of small redox proteins known to be present in all organisms. It plays a role in many important biological processes, including redox signaling. In humans, thioredoxins are encoded by ''TXN'' and ''TXN2'' genes ...
regulatory chain. It catalyzes the following reaction:
::: 2 reduced ferredoxin
Ferredoxins (from Latin ''ferrum'': iron + redox, often abbreviated "fd") are iron–sulfur proteins that mediate electron transfer in a range of metabolic reactions. The term "ferredoxin" was coined by D.C. Wharton of the DuPont Co. and applied t ...
+ thioredoxin
Thioredoxin (TRX or TXN) is a class of small redox proteins known to be present in all organisms. It plays a role in many important biological processes, including redox signaling. In humans, thioredoxins are encoded by ''TXN'' and ''TXN2'' genes ...
disulfide
In chemistry, a disulfide (or disulphide in British English) is a compound containing a functional group or the anion. The linkage is also called an SS-bond or sometimes a disulfide bridge and usually derived from two thiol groups.
In inorg ...
2 oxidized ferredoxin + thioredoxin thiols + 2 H+
Ferredoxin-Thioredoxin reductase (FTR) converts an electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
signal (photoreduced ferredoxin) to a thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl grou ...
signal (reduced thioredoxin), regulating enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
s by reduction of specific disulfide
In chemistry, a disulfide (or disulphide in British English) is a compound containing a functional group or the anion. The linkage is also called an SS-bond or sometimes a disulfide bridge and usually derived from two thiol groups.
In inorg ...
groups. It catalyses the light-dependent activation of several photosynthesis
Photosynthesis ( ) is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabo ...
enzymes
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as pro ...
and constitutes the first historical example of a thiol/disulfide exchange cascade for enzyme regulation. It is a heterodimer
In biochemistry, a protein dimer is a macromolecular complex or multimer formed by two protein monomers, or single proteins, which are usually non-covalently bound. Many macromolecules, such as proteins or nucleic acids, form dimers. The word ...
of subunit alpha and subunit beta. Subunit alpha is the variable subunit, and beta is the catalytic
Catalysis () is the increase in reaction rate, rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst ...
chain. The structure
A structure is an arrangement and organization of interrelated elements in a material object or system, or the object or system so organized. Material structures include man-made objects such as buildings and machines and natural objects such as ...
of the beta subunit has been determined and found to fold around the FeS cluster.
Biological Function
Major groups of oxygen-producing,photosynthetic
Photosynthesis ( ) is a Biological system, system of biological processes by which Photoautotrophism, photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical ener ...
organisms such as cyanobacteria
Cyanobacteria ( ) are a group of autotrophic gram-negative bacteria that can obtain biological energy via oxygenic photosynthesis. The name "cyanobacteria" () refers to their bluish green (cyan) color, which forms the basis of cyanobacteri ...
, algae
Algae ( , ; : alga ) is an informal term for any organisms of a large and diverse group of photosynthesis, photosynthetic organisms that are not plants, and includes species from multiple distinct clades. Such organisms range from unicellular ...
, C4, C3, and crassulacean acid metabolism (CAM) plants use Ferredoxin-thioredoxin reductase for carbon fixation
Biological carbon fixation, or сarbon assimilation, is the Biological process, process by which living organisms convert Total inorganic carbon, inorganic carbon (particularly carbon dioxide, ) to Organic compound, organic compounds. These o ...
regulation. FTR, as part of a greater Ferredoxin-Thioredoxin system, allows plants to change their metabolism based on light intensity. Specifically, the Ferredoxin-Thioredoxin system controls enzymes in the Calvin Cycle
The Calvin cycle, light-independent reactions, bio synthetic phase, dark reactions, or photosynthetic carbon reduction (PCR) cycle of photosynthesis is a series of chemical reactions that convert carbon dioxide and hydrogen-carrier compounds into ...
and Pentose phosphate pathway
The pentose phosphate pathway (also called the phosphogluconate pathway and the hexose monophosphate shunt or HMP shunt) is a metabolic pathway parallel to glycolysis. It generates NADPH and pentoses (five-carbon sugars) as well as ribose 5-ph ...
- allowing plants to balance carbohydrate synthesis and degradation based on the availability of light. In the light, photosynthesis harnesses light energy and reduces Ferredoxin
Ferredoxins (from Latin ''ferrum'': iron + redox, often abbreviated "fd") are iron–sulfur proteins that mediate electron transfer in a range of metabolic reactions. The term "ferredoxin" was coined by D.C. Wharton of the DuPont Co. and applied t ...
. Using FTR, reduced Ferredoxin then reduces Thioredoxin
Thioredoxin (TRX or TXN) is a class of small redox proteins known to be present in all organisms. It plays a role in many important biological processes, including redox signaling. In humans, thioredoxins are encoded by ''TXN'' and ''TXN2'' genes ...
. Thioredoxin, through thiol/disulfide exchange, then activates carbohydrate synthesis enzymes such as chloroplast fructose-1,6-bisphosphatase, Sedoheptulose-bisphosphatase, and phosphoribulokinase. As a result, light uses FTR to activate carbohydrate biosynthesis. In the dark, Ferredoxin remains oxidized. This leaves Thioredoxin inactive and allows carbohydrate breakdown to dominate metabolism.
Structure
Ferredoxin-Thioredoxin Reductase is an α-βheterodimer
In biochemistry, a protein dimer is a macromolecular complex or multimer formed by two protein monomers, or single proteins, which are usually non-covalently bound. Many macromolecules, such as proteins or nucleic acids, form dimers. The word ...
of approximately 30 kDa. FTR structure across different plant species include a conserved catalytic β subunit and a variable α subunit. The structure of FTR from '' Synechocystis'' sp. PCC6803 has been studied in detail and resolved at 1.6 Å. FTR resembles a thin concave disc, 10 Å across the center where a Fe-4S clusterresides. One side of the cluster center contains redox-active disulfide bonds that reduce Thioredoxin while the opposite docks with reduced Ferredoxin. This two sided disc structure allows FTR to simultaneously interact with Thioredoxin and Ferredoxin.
 The variable α subunit has an open β barrel structure made of five antiparallel β strands. Its interaction with the catalytic subunit occurs mainly with two loops between β strands. The residues in these two loops are mostly conserved and are thought to stabilize the 4Fe-4S cluster in the catalytic subunit. Structurally, the α subunit is very similar to the PsaE protein, a subunit of
The variable α subunit has an open β barrel structure made of five antiparallel β strands. Its interaction with the catalytic subunit occurs mainly with two loops between β strands. The residues in these two loops are mostly conserved and are thought to stabilize the 4Fe-4S cluster in the catalytic subunit. Structurally, the α subunit is very similar to the PsaE protein, a subunit of Photosystem I
Photosystem I (PSI, or plastocyanin–ferredoxin oxidoreductase) is one of two photosystems in the Light-dependent reactions, photosynthetic light reactions of algae, plants, and cyanobacteria. Photosystem I is an integral membrane ...
, though the similarity is not seen in their sequences or functions.
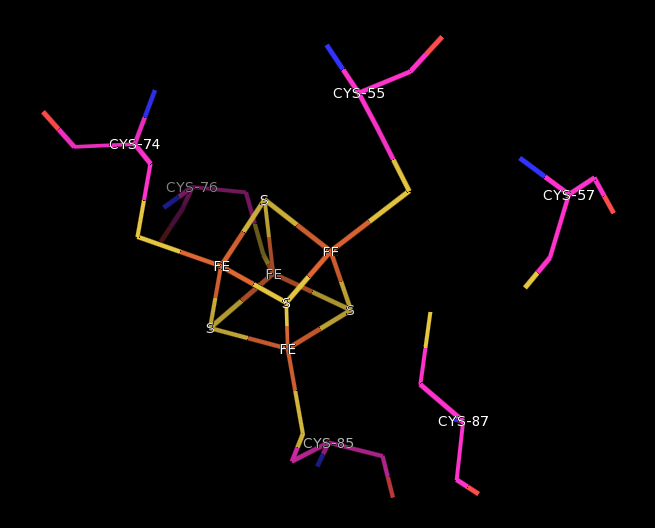
The catalytic β subunit has a general α-helical structure with an Fe-4S center The FeS center and redox-active Cysteine
Cysteine (; symbol Cys or C) is a semiessential proteinogenic amino acid with the chemical formula, formula . The thiol side chain in cysteine enables the formation of Disulfide, disulfide bonds, and often participates in enzymatic reactions as ...
residues are located within the loops of these helices. Cysteine-55, 74, 76, and 85 are coordinated to the iron atoms of the cubane-type cluster.
Enzymatic Mechanism
FTR is ''unique'' amongthioredoxin reductase
Thioredoxin reductases (TR, TrxR) () are enzymes that reduce thioredoxin (Trx). Two classes of thioredoxin reductase have been identified: one class in bacteria and some eukaryotes and one in animals. Bacterial TrxR also catalyzes the reduction ...
s because it uses an Fe-S cluster cofactor rather than flavoprotein
Flavoproteins are proteins that contain a nucleic acid derivative of riboflavin. These proteins are involved in a wide array of biological processes, including removal of radicals contributing to oxidative stress, photosynthesis, and DNA repair. ...
s to reduce disulfide bonds. FTR catalysis begins with its interaction with reduced Ferredoxin. This proceeds with the attraction between FTR Lys-47 and Ferredoxin Glu-92. One electron from Ferredoxin and one electron from the Fe-S center is abstracted to break FTR's Cys-87 and Cys-57 disulfide bond, create a nucleophilic Cys-57, and oxidize the Fe-S center from Fe-4Ssup>2+ to Fe-4Ssup>3+. The structure of this one-electron (from Ferredoxin) intermediate is contested: Staples et al. suggest Cys-87 is coordinated to a Sulfur in the Fe-S center while Dai et al. argue Cys-87 is coordinated to an Iron. Next, the nucleophilic Cys-57, encouraged by an adjacent Histidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an Amine, α-amino group (which is in the protonated –NH3+ form under Physiological condition, biological conditions), a carboxylic ...
residue, attacks a disulfide bridge on Thioredoxin, creating a hetero-disulfide Thioredoxin intermediate. Lastly, a newly docked Ferredoxin molecule delivers the final electron to the FeS center, reducing it to its original 2+ state, reforming the Cys-87, Cys-57 disulfide, and fully reducing thioredoxin to two thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl grou ...
s.
References
External links
* {{Portal bar, Biology, border=no Protein domains EC 1.8.7