electron-withdrawing group on:
[Wikipedia]
[Google]
[Amazon]
An electron-withdrawing group (EWG) is a
 The inductive effect is cumulative:
The inductive effect is cumulative: 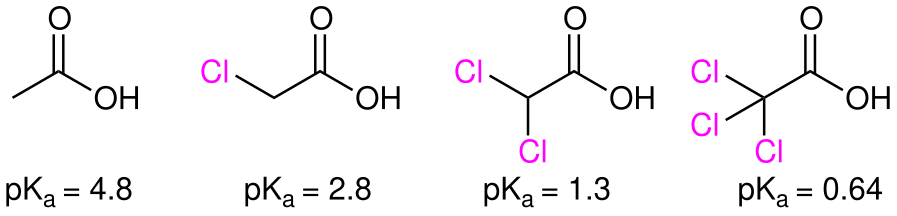 :The impact of the EWG on pKa decreases with distances from the carboxylic group.
:The impact of the EWG on pKa decreases with distances from the carboxylic group.
 For benzoic acids, the effect is quantified by the Hammett equation:
:
where
: = Reference constant
: = Substituent constant
: =
For benzoic acids, the effect is quantified by the Hammett equation:
:
where
: = Reference constant
: = Substituent constant
: =
group
A group is a number of persons or things that are located, gathered, or classed together.
Groups of people
* Cultural group, a group whose members share the same cultural identity
* Ethnic group, a group whose members share the same ethnic iden ...
or atom that has the ability to draw electron density toward itself and away from other adjacent atoms. This electron density transfer is often achieved by resonance or inductive effects. Electron-withdrawing groups have significant impacts on fundamental chemical processes such as acid-base reactions, redox potential
Redox potential (also known as oxidation / reduction potential, ''ORP'', ''pe'', ''E_'', or E_) is a measure of the tendency of a chemical species to acquire electrons from or lose electrons to an electrode and thereby be reduced or oxidised respe ...
s, and substitution reactions.
Consequences of EWGs
Effects on Brønsted–Lowry acidity
Electron-withdrawing groups exert an " inductive" or "electron-pulling" effect oncovalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
s. The strength of the electron-withdrawing group is inversely proportional to the pKa
In chemistry, an acid dissociation constant (also known as acidity constant, or acid-ionization constant; denoted ) is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction
:H ...
of the carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
.
: The inductive effect is cumulative:
The inductive effect is cumulative: trichloroacetic acid
Trichloroacetic acid (TCA; TCAA; also known as trichloroethanoic acid) is an analogue of acetic acid in which the three hydrogen atoms of the methyl group have all been replaced by chlorine atoms. Salts and esters of trichloroacetic acid are cal ...
is 1000× stronger than chloroacetic acid
Chloroacetic acid, industrially known as monochloroacetic acid (MCA), is the organochlorine compound with the formula . This carboxylic acid is a useful building block in organic synthesis. It is a colorless solid. Related compounds are dichlo ...
.
: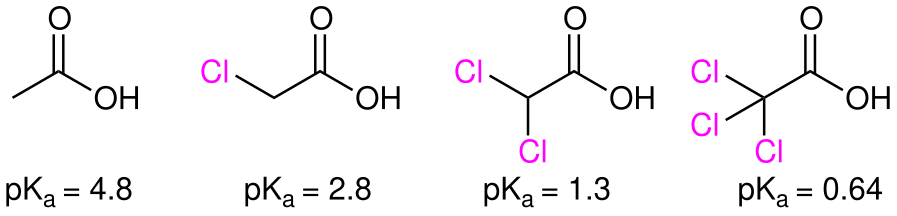 :The impact of the EWG on pKa decreases with distances from the carboxylic group.
:The impact of the EWG on pKa decreases with distances from the carboxylic group.
 For benzoic acids, the effect is quantified by the Hammett equation:
:
where
: = Reference constant
: = Substituent constant
: =
For benzoic acids, the effect is quantified by the Hammett equation:
:
where
: = Reference constant
: = Substituent constant
: = Reaction rate constant
In chemical kinetics, a reaction rate constant or reaction rate coefficient () is a proportionality constant which quantifies the rate and direction of a chemical reaction by relating it with the concentration of reactants.
For a reaction between ...
Effect on Lewis acidity
Electron-withdrawing groups tend to lower Lewis basicity. EWGs enhance theLewis acidity
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty Non-bonding orbital, orbital which is capable of accepting an electron pair from a Lewis Base (chemistry), base to form a Lewis ...
, making compounds more reactive as Lewis acids
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
. For example, fluorine is a stronger electron-withdrawing substituent than methyl
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula (whereas normal methane has the formula ). In formulas, the group is often abbreviated as ...
, resulting in an increased Lewis acidity
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty Non-bonding orbital, orbital which is capable of accepting an electron pair from a Lewis Base (chemistry), base to form a Lewis ...
of boron trifluoride
Boron trifluoride is the inorganic compound with the formula . This pungent, colourless, and toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.
Structure and bonding
The g ...
relative to trimethylborane
Trimethylborane (TMB) is a toxic, pyrophoric gas with the formula B(CH3)3 (which can also be written as Me3B, with Me representing methyl).
Properties
As a liquid it is colourless. The strongest line in the infrared spectrum is at 1330 cm� ...
.
This effect of EWG has been quantified in many of ways. The Tolman electronic parameter
The Tolman electronic parameter (TEP) is a measure of the electron donating or withdrawing ability of a ligand. It is determined by measuring the frequency of the A1 C-O vibrational mode (ν(CO)) of a (pseudo)-C3v Molecular symmetry, symmetric comp ...
is determined by the frequency of a C-O vibrational mode (ν(CO)) of the coordination complex
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ' ...
es Ni(CO)3(L = Lewis base).
Effect on a aromatic substitution reactions

Electrophilic aromatic substitution
Electrophilic aromatic substitution (SEAr) is an organic reaction in which an atom that is attached to an aromatic ring, aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitut ...
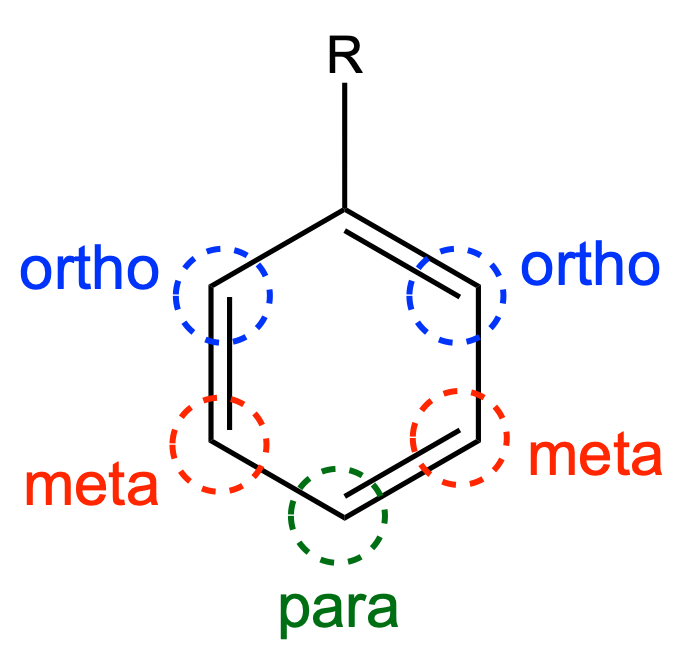
is famously affected by EWGs. The effect is transmitted by inductive and resonance effects. Benzene with an EWG typically undergoes electrophilic substitution at meta positions. Overall the rates are diminished. thus EWGs are called deactivating.
When it comes to nucleophilic substitution reactions, electron-withdrawing groups are more prone to nucleophilic substitution
In chemistry, a nucleophilic substitution (SN) is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile) ...
. For example, chlorodinitrobenzene is far more susceptible to reactions displacing chloride compared to chlorobenzene
Chlorobenzene (abbreviated PhCl) is an aryl chloride and the simplest of the chlorobenzenes, consisting of a benzene ring substituted with one chlorine atom. Its chemical formula is C6H5Cl. This colorless, flammable liquid is a common solvent a ...
.
Effects on redox potential
In the context ofelectron transfer
Electron transfer (ET) occurs when an electron relocates from an atom, ion, or molecule, to another such chemical entity. ET describes the mechanism by which electrons are transferred in redox reactions.
Electrochemical processes are ET reactio ...
, these groups enhance the oxidizing power tendency of the attached species. For example, Tetracyanoethylene
Tetracyanoethylene (TCNE) is organic compound with the formula . It is a colorless solid, although samples are often off-white. It is an important member of the cyanocarbons.
Synthesis and reactions
TCNE is prepared by brominating malononitril ...
serves as an oxidant due to its four cyano substituents, which are electron-withdrawing.
Oxidants with EWGs are stronger than the parent compound. Acetylferrocenium is 300 mV more oxidizing than ferrocene
Ferrocene is an organometallic chemistry, organometallic compound with the formula . The molecule is a Cyclopentadienyl complex, complex consisting of two Cyclopentadienyl anion, cyclopentadienyl rings sandwiching a central iron atom. It is an o ...
.
Comparison with electron-donating groups
Electron-withdrawing groups are the opposite effect of electron-donating groups (EDGs). Both describefunctional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
s, however, electron-withdrawing groups pull electron density
Electron density or electronic density is the measure of the probability of an electron being present at an infinitesimal element of space surrounding any given point. It is a scalar quantity depending upon three spatial variables and is typical ...
away from a molecule, whereas EDGs push electron density onto a substituent.{{Cite web , last=Hunt , first=Ian , date=2023-10-22 , title=Chapter 12: Reactions of Arenes. Electrophilic Aromatic Substitution , url=https://www.chem.ucalgary.ca/courses/350/Carey5th/Ch12/ch12-8b.html
See also
* Electron-donating groupReferences