Electromagnetic Absorption By Water on:
[Wikipedia]
[Google]
[Amazon]
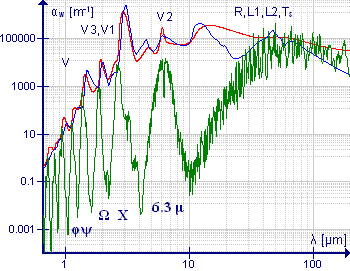 The absorption of electromagnetic radiation by water depends on the
The absorption of electromagnetic radiation by water depends on the

Rydberg series: 1''b''1 (n2) → many different Rydberg states and 3''a''1 (n1) → 3''sa''1 Rydberg state **128 nm band
Rydberg series: 3''a''1 (n1) → 3''sa''1 Rydberg state and 1''b''1 (n2) → 3s''a''1 Rydberg state **166.5 nm band
1''b''1 (n2) → 4''a''1 (σ1*-like orbital)
 Water vapor is a
Water vapor is a
High resolution gas-phase absorption simulations
(archived version) {{Water Water physics Chemical physics Absorption spectroscopy Electrochemistry Electric and magnetic fields in matter
 The absorption of electromagnetic radiation by water depends on the
The absorption of electromagnetic radiation by water depends on the state
State most commonly refers to:
* State (polity), a centralized political organization that regulates law and society within a territory
**Sovereign state, a sovereign polity in international law, commonly referred to as a country
**Nation state, a ...
of the water.
The absorption in the gas phase occurs in three regions of the spectrum. Rotational transitions are responsible for absorption in the microwave
Microwave is a form of electromagnetic radiation with wavelengths shorter than other radio waves but longer than infrared waves. Its wavelength ranges from about one meter to one millimeter, corresponding to frequency, frequencies between 300&n ...
and far-infrared, vibrational transitions in the mid-infrared and near-infrared
Infrared (IR; sometimes called infrared light) is electromagnetic radiation (EMR) with wavelengths longer than that of visible light but shorter than microwaves. The infrared spectral band begins with the waves that are just longer than those of ...
. Vibrational bands have rotational fine structure. Electronic transitions occur in the vacuum ultraviolet regions.
Its weak absorption in the visible spectrum
The visible spectrum is the spectral band, band of the electromagnetic spectrum that is visual perception, visible to the human eye. Electromagnetic radiation in this range of wavelengths is called ''visible light'' (or simply light).
The optica ...
results in the pale blue color of water
The color of water varies with the ambient conditions in which that water is present. While relatively small quantities of water appear to be colorless, pure water has a slight blue color that becomes deeper as the thickness of the observed s ...
.
Overview
The water molecule, in the gaseous state, has three types of transition that can give rise to absorption of electromagnetic radiation: * Rotational transitions, in which the molecule gains a quantum ofrotational energy
Rotational energy or angular kinetic energy is kinetic energy due to the rotation of an object and is part of its total kinetic energy. Looking at rotational energy separately around an object's axis of rotation, the following dependence on the ob ...
. Atmospheric water vapour at ambient temperature and pressure gives rise to absorption in the far-infrared region of the spectrum, from about 200 cm−1 (50 μm) to longer wavelengths towards the microwave
Microwave is a form of electromagnetic radiation with wavelengths shorter than other radio waves but longer than infrared waves. Its wavelength ranges from about one meter to one millimeter, corresponding to frequency, frequencies between 300&n ...
region.
* Vibrational transitions in which a molecule gains a quantum of vibrational energy. The fundamental transitions give rise to absorption in the mid-infrared in the regions around 1650 cm−1 (μ band, 6 μm) and 3500 cm−1 (so-called X band, 2.9 μm)
* Electronic transitions in which a molecule is promoted to an excited electronic state. The lowest energy transition of this type is in the vacuum ultraviolet region.
In reality, vibrations of molecules in the gaseous state are accompanied by rotational transitions, giving rise to a vibration-rotation spectrum. Furthermore, vibrational overtone
An overtone is any resonant frequency above the fundamental frequency of a sound. (An overtone may or may not be a harmonic) In other words, overtones are all pitches higher than the lowest pitch within an individual sound; the fundamental i ...
s and combination bands occur in the near-infrared
Infrared (IR; sometimes called infrared light) is electromagnetic radiation (EMR) with wavelengths longer than that of visible light but shorter than microwaves. The infrared spectral band begins with the waves that are just longer than those of ...
region. The HITRAN spectroscopy
Spectroscopy is the field of study that measures and interprets electromagnetic spectra. In narrower contexts, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum.
Spectro ...
database lists more than 37,000 spectral line
A spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. It may result from emission (electromagnetic radiation), emission or absorption (electromagnetic radiation), absorption of light in a narrow frequency ...
s for gaseous H216O, ranging from the microwave
Microwave is a form of electromagnetic radiation with wavelengths shorter than other radio waves but longer than infrared waves. Its wavelength ranges from about one meter to one millimeter, corresponding to frequency, frequencies between 300&n ...
region to the visible spectrum
The visible spectrum is the spectral band, band of the electromagnetic spectrum that is visual perception, visible to the human eye. Electromagnetic radiation in this range of wavelengths is called ''visible light'' (or simply light).
The optica ...
.
In liquid water the rotational transitions are effectively quenched, but absorption bands are affected by hydrogen bonding
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, Covalent bond, covalently b ...
. In crystalline ice the vibrational spectrum is also affected by hydrogen bonding and there are lattice vibration
A phonon is a collective excitation in a periodic, elastic arrangement of atoms or molecules in condensed matter, specifically in solids and some liquids. In the context of optically trapped objects, the quantized vibration mode can be defined ...
s causing absorption in the far-infrared. Electronic transitions of gaseous molecules will show both vibrational and rotational fine structure.
Units
Infrared absorption band positions may be given either inwavelength
In physics and mathematics, wavelength or spatial period of a wave or periodic function is the distance over which the wave's shape repeats.
In other words, it is the distance between consecutive corresponding points of the same ''phase (waves ...
(usually in micrometers, μm) or wavenumber
In the physical sciences, the wavenumber (or wave number), also known as repetency, is the spatial frequency of a wave. Ordinary wavenumber is defined as the number of wave cycles divided by length; it is a physical quantity with dimension of ...
(usually in reciprocal centimeters, cm−1) scale.
Rotational spectrum
The water molecule is an asymmetric top, that is, it has three independent moments of inertia. Rotation about the 2-foldsymmetry axis
Axial symmetry is symmetry around an axis or line (geometry). An object is said to be ''axially symmetric'' if its appearance is unchanged if transformed around an axis. The main types of axial symmetry are ''reflection symmetry'' and ''rotatio ...
is illustrated at the left. Because of the low symmetry of the molecule, a large number of transitions can be observed in the far infrared
Far infrared (FIR) or long wave refers to a specific range within the infrared spectrum of electromagnetic radiation. It encompasses radiation with wavelengths ranging from 15 μm ( micrometers) to 1 mm, which corresponds to a freque ...
region of the spectrum. Measurements of microwave spectra have provided a very precise value for the O−H bond length
In molecular geometry, bond length or bond distance is defined as the average distance between Atomic nucleus, nuclei of two chemical bond, bonded atoms in a molecule. It is a Transferability (chemistry), transferable property of a bond between at ...
, 95.84 ± 0.05 pm and H−O−H bond angle, 104.5 ± 0.3°.
Vibrational spectrum
The water molecule has three fundamentalmolecular vibration
A molecular vibration is a Periodic function, periodic motion of the atoms of a molecule relative to each other, such that the center of mass of the molecule remains unchanged. The infrared spectroscopy correlation table, typical vibrational fre ...
s. The O-H stretching vibrations give rise to absorption bands with band origins at 3657 cm−1 (ν1, 2.734 μm) and 3756 cm−1 (ν3, 2.662 μm) in the gas phase. The asymmetric stretching vibration, of B2 symmetry in the point group
In geometry, a point group is a group (mathematics), mathematical group of symmetry operations (isometry, isometries in a Euclidean space) that have a Fixed point (mathematics), fixed point in common. The Origin (mathematics), coordinate origin o ...
C2v is a normal vibration. The H-O-H bending mode origin is at 1595 cm−1 (ν2, 6.269 μm). Both symmetric stretching and bending vibrations have A1 symmetry, but the frequency difference between them is so large that mixing is effectively zero. In the gas phase all three bands show extensive rotational fine structure. In the near-infrared spectrum ν3 has a series of overtones
An overtone is any resonant frequency above the fundamental frequency of a sound. (An overtone may or may not be a harmonic) In other words, overtones are all pitches higher than the lowest pitch within an individual sound; the fundamental i ...
at wavenumbers somewhat less than n·ν3, n=2,3,4,5... Combination bands, such as ν2 + ν3 are also easily observed in the near-infrared region. The presence of water vapor
Water vapor, water vapour, or aqueous vapor is the gaseous phase of Properties of water, water. It is one Phase (matter), state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from th ...
in the atmosphere is important for atmospheric chemistry
Atmospheric chemistry is a branch of atmospheric science that studies the chemistry of the Earth's atmosphere and that of other planets. This multidisciplinary approach of research draws on environmental chemistry, physics, meteorology, comput ...
especially as the infrared and near infrared spectra are easy to observe. Standard (atmospheric optical) codes are assigned to absorption bands as follows. 0.718 μm (visible): α, 0.810 μm: μ, 0.935 μm: ρστ, 1.13 μm: φ, 1.38 μm: ψ, 1.88 μm: Ω, 2.68 μm: X. The gaps between the bands define the infrared window
The infrared atmospheric window is an atmospheric window in the infrared spectrum where there is relatively little absorption of terrestrial thermal radiation by atmospheric gases. The window plays an important role in the atmospheric greenhouse ...
in the Earth's atmosphere.
The infrared spectrum of liquid water is dominated by the intense absorption due to the fundamental O-H stretching vibrations. Because of the high intensity, very short path lengths, usually less than 50 μm, are needed to record the spectra of aqueous solutions. There is no rotational fine structure, but the absorption bands are broader than might be expected, because of hydrogen bonding
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, Covalent bond, covalently b ...
. Peak maxima for liquid water are observed at 3450 cm−1 (2.898 μm), 3615 cm−1 (2.766 μm) and 1640 cm −1 (6.097 μm). Direct measurement of the infrared spectra of aqueous solutions requires that the cuvette windows be made of substances such as calcium fluoride which are water-insoluble. This difficulty can alternatively be overcome by using an attenuated total reflectance (ATR) device rather than transmission.
In the near-infrared range liquid water has absorption bands around 1950 nm (5128 cm−1), 1450 nm (6896 cm−1), 1200 nm (8333 cm−1) and 970 nm, (10300 cm−1). The regions between these bands can be used in near-infrared spectroscopy
Near-infrared spectroscopy (NIRS) is a spectroscopic method that uses the near-infrared region of the electromagnetic spectrum (from 780 nm to 2500 nm). Typical applications include medical and physiological diagnostics and research inc ...
to measure the spectra of aqueous solutions, with the advantage that glass is transparent in this region, so glass cuvettes can be used. The absorption intensity is weaker than for the fundamental vibrations, but this is not important as longer path-length cuvettes can be used. The absorption band at 698 nm (14300 cm−1) is a 3rd overtone (n=4). It tails off onto the visible region and is responsible for the intrinsic blue color of water
The color of water varies with the ambient conditions in which that water is present. While relatively small quantities of water appear to be colorless, pure water has a slight blue color that becomes deeper as the thickness of the observed s ...
. This can be observed with a standard UV/vis spectrophotometer, using a 10 cm path-length. The colour can be seen by eye by looking through a column of water about 10 m in length; the water must be passed through an ultrafilter
In the Mathematics, mathematical field of order theory, an ultrafilter on a given partially ordered set (or "poset") P is a certain subset of P, namely a Maximal element, maximal Filter (mathematics), filter on P; that is, a proper filter on P th ...
to eliminate color due to Rayleigh scattering
Rayleigh scattering ( ) is the scattering or deflection of light, or other electromagnetic radiation, by particles with a size much smaller than the wavelength of the radiation. For light frequencies well below the resonance frequency of the scat ...
which also can make water appear blue.
The spectrum of ice is similar to that of liquid water, with peak maxima at 3400 cm−1 (2.941 μm), 3220 cm−1 (3.105 μm) and 1620 cm−1 (6.17 μm)
In both liquid water and ice clusters, low-frequency vibrations occur, which involve the stretching (TS) or bending (TB) of intermolecular hydrogen bonds (O–H•••O). Bands at wavelengths λ = 50-55 μm or 182-200 cm−1 (44 μm, 227 cm−1 in ice) have been attributed to TS, intermolecular stretch, and 200 μm or 50 cm−1 (166 μm, 60 cm−1 in ice), to TB, intermolecular bend
Visible region
Absorption coefficients for 200 nm and 900 nm are almost equal at 6.9 m−1 ( attenuation length of 14.5 cm). Very weak light absorption, in the visible region, by liquid water has been measured using an integrating cavity absorption meter (ICAM). The absorption was attributed to a sequence of overtone and combination bands whose intensity decreases at each step, giving rise to an absolute minimum at 418 nm, at which wavelength the attenuation coefficient is about 0.0044 m−1, which is an attenuation length of about 227 meters. These values correspond to pure absorption without scattering effects. The attenuation of, e.g., a laser beam would be slightly stronger.Electronic spectrum
The electronic transitions of the water molecule lie in the vacuum ultraviolet region. For water vapor the bands have been assigned as follows. *65 nm band — many different electronic transitions,photoionization
Photoionization is the physical process in which an ion is formed from the interaction of a photon with an atom or molecule.
Cross section
Not every interaction between a photon and an atom, or molecule, will result in photoionization. The prob ...
, photodissociation
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by absorption of light or photons. It is defined as the interaction of one or more photons wi ...
*discrete features between 115 and 180 nm
**set of narrow bands between 115 and 125 nmRydberg series: 1''b''1 (n2) → many different Rydberg states and 3''a''1 (n1) → 3''sa''1 Rydberg state **128 nm band
Rydberg series: 3''a''1 (n1) → 3''sa''1 Rydberg state and 1''b''1 (n2) → 3s''a''1 Rydberg state **166.5 nm band
1''b''1 (n2) → 4''a''1 (σ1*-like orbital)
Microwaves and radio waves
The pure rotation spectrum of water vapor extends into the microwave region. Liquid water has a broad absorption spectrum in the microwave region, which has been explained in terms of changes in thehydrogen bond
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, Covalent bond, covalently b ...
network giving rise to a broad, featureless, microwave spectrum. The absorption (equivalent to dielectric loss
In electrical engineering, dielectric loss quantifies a dielectric material's inherent dissipation of electromagnetic energy (e.g. heat). It can be parameterized in terms of either the loss angle or the corresponding loss tangent . Both refer ...
) is used in microwave oven
A microwave oven, or simply microwave, is an electric oven that heats and cooks food by exposing it to electromagnetic radiation in the microwave frequency range. This induces Dipole#Molecular dipoles, polar molecules in the food to rotate and ...
s to heat food that contains water molecules. A frequency of 2.45 GHz, wavelength 122 mm, is commonly used.
Radiocommunication at GHz frequencies is very difficult in fresh waters and even more so in salt waters.
Atmospheric effects
 Water vapor is a
Water vapor is a greenhouse gas
Greenhouse gases (GHGs) are the gases in the atmosphere that raise the surface temperature of planets such as the Earth. Unlike other gases, greenhouse gases absorb the radiations that a planet emits, resulting in the greenhouse effect. T ...
in the Earth's atmosphere
The atmosphere of Earth is composed of a layer of gas mixture that surrounds the Earth's planetary surface (both lands and oceans), known collectively as air, with variable quantities of suspended aerosols and particulates (which create weathe ...
, responsible for 70% of the known absorption of incoming sunlight
Sunlight is the portion of the electromagnetic radiation which is emitted by the Sun (i.e. solar radiation) and received by the Earth, in particular the visible spectrum, visible light perceptible to the human eye as well as invisible infrare ...
, particularly in the infrared region, and about 60% of the atmospheric absorption of thermal radiation
Thermal radiation is electromagnetic radiation emitted by the thermal motion of particles in matter. All matter with a temperature greater than absolute zero emits thermal radiation. The emission of energy arises from a combination of electro ...
by the Earth known as the greenhouse effect
The greenhouse effect occurs when greenhouse gases in a planet's atmosphere insulate the planet from losing heat to space, raising its surface temperature. Surface heating can happen from an internal heat source (as in the case of Jupiter) or ...
. It is also an important factor in multispectral imaging and hyperspectral imaging
Hyperspectral imaging collects and processes information from across the electromagnetic spectrum. The goal of hyperspectral imaging is to obtain the spectrum for each pixel in the image of a scene, with the purpose of finding objects, identifyi ...
used in remote sensing
Remote sensing is the acquisition of information about an physical object, object or phenomenon without making physical contact with the object, in contrast to in situ or on-site observation. The term is applied especially to acquiring inform ...
because water vapor absorbs radiation differently in different spectral bands. Its effects are also an important consideration in infrared astronomy
Infrared astronomy is a sub-discipline of astronomy which specializes in the astronomical observation, observation and analysis of astronomical objects using infrared (IR) radiation. The wavelength of infrared light ranges from 0.75 to 300 microm ...
and radio astronomy
Radio astronomy is a subfield of astronomy that studies Astronomical object, celestial objects using radio waves. It started in 1933, when Karl Jansky at Bell Telephone Laboratories reported radiation coming from the Milky Way. Subsequent observat ...
in the microwave
Microwave is a form of electromagnetic radiation with wavelengths shorter than other radio waves but longer than infrared waves. Its wavelength ranges from about one meter to one millimeter, corresponding to frequency, frequencies between 300&n ...
or millimeter wave
Extremely high frequency (EHF) is the International Telecommunication Union designation for the band of radio frequencies in the electromagnetic spectrum from 30 to 300 gigahertz (GHz). It is in the microwave part of the radio spectrum, between t ...
bands. The South Pole Telescope was constructed in Antarctica
Antarctica () is Earth's southernmost and least-populated continent. Situated almost entirely south of the Antarctic Circle and surrounded by the Southern Ocean (also known as the Antarctic Ocean), it contains the geographic South Pole. ...
in part because the elevation and low temperatures there mean there is very little water vapor in the atmosphere.
Similarly, carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
absorption bands occur around 1400, 1600 and 2000 nm, but its presence in the Earth's atmosphere accounts for just 26% of the greenhouse effect. Carbon dioxide gas absorbs energy in some small segments of the thermal infrared spectrum that water vapor misses. This extra absorption within the atmosphere causes the air to warm just a bit more and the warmer the atmosphere the greater its capacity to hold more water vapor. This extra water vapor absorption further enhances the Earth's greenhouse effect.
In the atmospheric window between approximately 8000 and 14000 nm, in the far-infrared spectrum, carbon dioxide and water absorption is weak. This window allows most of the thermal radiation in this band to be radiated out to space directly from the Earth's surface. This band is also used for remote sensing of the Earth from space, for example with thermal Infrared
Infrared (IR; sometimes called infrared light) is electromagnetic radiation (EMR) with wavelengths longer than that of visible light but shorter than microwaves. The infrared spectral band begins with the waves that are just longer than those ...
imaging.
As well as absorbing radiation, water vapour occasionally emits radiation in all directions, according to the Black Body
A black body or blackbody is an idealized physical body that absorbs all incident electromagnetic radiation, regardless of frequency or angle of incidence. The radiation emitted by a black body in thermal equilibrium with its environment is ...
Emission curve for its current temperature overlaid on the water absorption spectrum. Much of this energy will be recaptured by other water molecules, but at higher altitudes, radiation sent towards space is less likely to be recaptured, as there is less water available to recapture radiation of water-specific absorbing wavelengths. By the top of the troposphere
The troposphere is the lowest layer of the atmosphere of Earth. It contains 80% of the total mass of the Atmosphere, planetary atmosphere and 99% of the total mass of water vapor and aerosols, and is where most weather phenomena occur. From the ...
, about 12 km above sea level, most water vapor condenses to liquid water or ice as it releases its heat of vapourization. Once changed state, liquid water and ice fall away to lower altitudes. This will be balanced by incoming water vapour rising via convection currents.
Liquid water and ice emit radiation at a higher rate than water vapour (see graph above). Water at the top of the troposphere, particularly in liquid and solid states, cools as it emits net photons to space. Neighboring gas molecules other than water (e.g. nitrogen) are cooled by passing their heat kinetically to the water. This is why temperatures at the top of the troposphere (known as the tropopause
The tropopause is the atmospheric boundary that demarcates the lowest two layers of the atmosphere of Earth – the troposphere and stratosphere – which occurs approximately above the equatorial regions, and approximately above the polar regi ...
) are about -50 degrees Celsius.
See also
* Dielectric spectroscopy * Differential optical absorption spectroscopy * Hydroxyl ion absorption inoptical fiber
An optical fiber, or optical fibre, is a flexible glass or plastic fiber that can transmit light from one end to the other. Such fibers find wide usage in fiber-optic communications, where they permit transmission over longer distances and at ...
* Water model
References
External links
High resolution gas-phase absorption simulations
(archived version) {{Water Water physics Chemical physics Absorption spectroscopy Electrochemistry Electric and magnetic fields in matter