Elbs Reaction on:
[Wikipedia]
[Google]
[Amazon]
The Elbs reaction is an
Organische Chemie: Grundlagen, Stoffklassen, Reaktionen, Konzepte, Molekülstrukturen
' (5th edition). Stuttgart: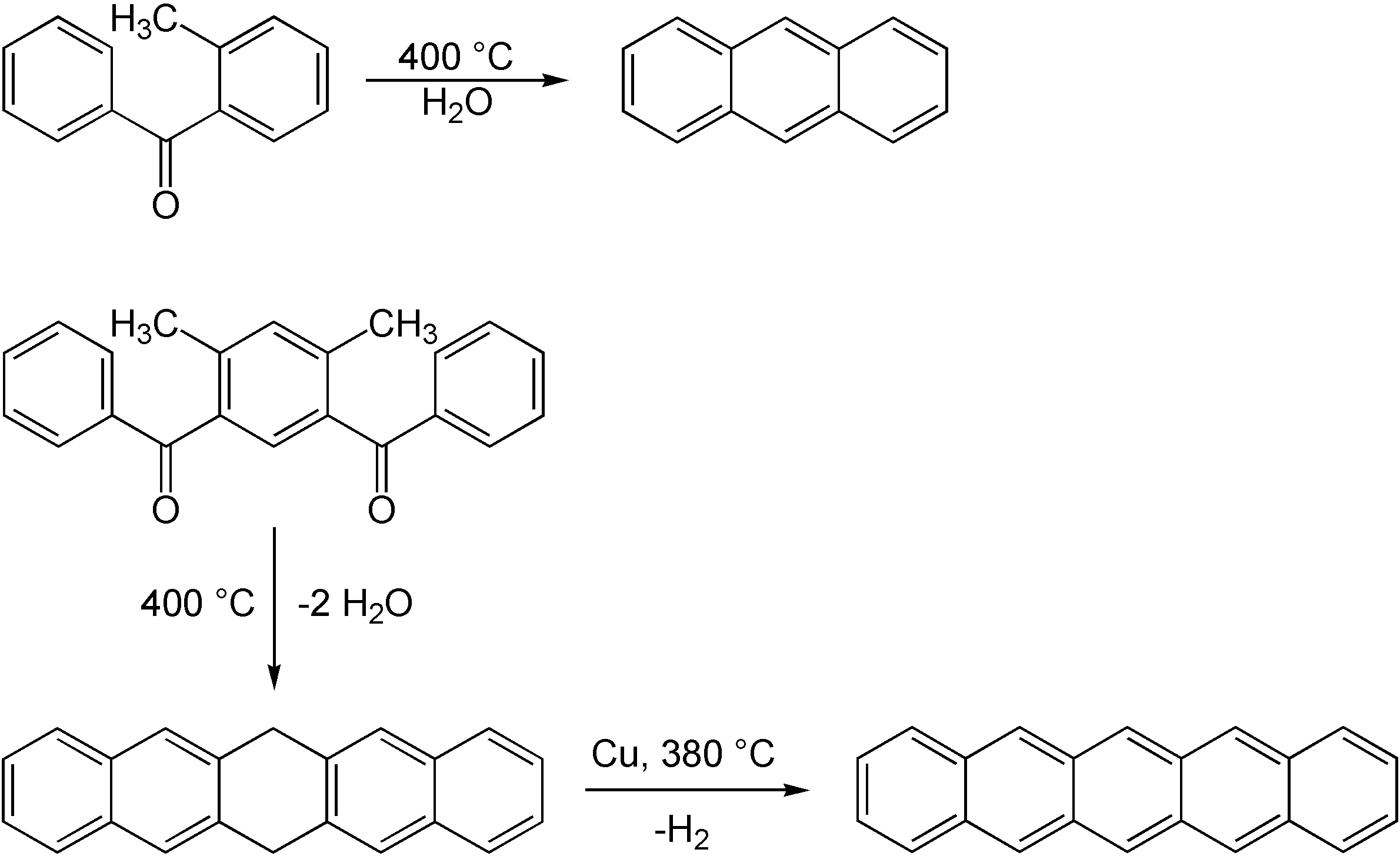 The
The
 Alternatively, in the second mechanism, due to Cook, the methylated aromatic compound instead first undergoes a
Alternatively, in the second mechanism, due to Cook, the methylated aromatic compound instead first undergoes a  A third mechanism has also been proposed, involving
A third mechanism has also been proposed, involving

organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, mechanistic organ ...
describing the pyrolysis
Pyrolysis is a process involving the Bond cleavage, separation of covalent bonds in organic matter by thermal decomposition within an Chemically inert, inert environment without oxygen. Etymology
The word ''pyrolysis'' is coined from the Gree ...
of an ortho methyl
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula (whereas normal methane has the formula ). In formulas, the group is often abbreviated as ...
substituted benzophenone
Benzophenone is a naturally occurring organic compound with the formula (C6H5)2CO, generally abbreviated Ph2CO. Benzophenone has been found in some fungi, fruits and plants, including grapes. It is a white solid with a low melting point and ros ...
to a condensed polyaromatic
A Polycyclic aromatic hydrocarbon (PAH) is any member of a class of organic compounds that is composed of multiple fused aromatic rings. Most are produced by the incomplete combustion of organic matter— by engine exhaust fumes, tobacco, incine ...
. The reaction is named after its inventor, the German chemist Karl Elbs, also responsible for the Elbs oxidation. The reaction was published in 1884. Karl Elbs, Einar Larsen. (1884). "Ueber Paraxylylphenylketon." '' Ber. Dtsch. Chem. Ges.'' (in German), 17(2): 2847–2849, . K. Elbs. (1886) "Beiträge zur Kenntniss aromatischer Ketone. Erste Mittheilung." '' J. Prakt. Chem.'' (in German), 33(1): 180–188, . Elbs however did not correctly interpret the reaction product due to a lack of knowledge about naphthalene
Naphthalene is an organic compound with formula . It is the simplest polycyclic aromatic hydrocarbon, and is a white Crystal, crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 Parts-per notation ...
structure.
Scope
The Elbs reaction enables the synthesis of condensed aromatic systems. As already demonstrated by Elbs in 1884 it is possible to obtainanthracene
Anthracene is a solid polycyclic aromatic hydrocarbon (PAH) of formula C14H10, consisting of three fused benzene rings. It is a component of coal tar. Anthracene is used in the production of the red dye alizarin and other dyes, as a scintil ...
through dehydration
In physiology, dehydration is a lack of total body water that disrupts metabolic processes. It occurs when free water loss exceeds intake, often resulting from excessive sweating, health conditions, or inadequate consumption of water. Mild deh ...
. Larger aromatic systems like pentacene
Pentacene () is a polycyclic aromatic hydrocarbon consisting of five linearly-fused benzene () rings. This highly conjugated compound is an organic semiconductor. The compound generates excitons upon absorption of ultra-violet ( UV) or visible ...
are also feasible. This reaction does not take place in a single step but leads first to dihydropentacene that is dehydrogenated in a second step with copper
Copper is a chemical element; it has symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orang ...
as a catalyst.Eberhard Breitmaier, Günther Jung (2005). Organische Chemie: Grundlagen, Stoffklassen, Reaktionen, Konzepte, Molekülstrukturen
' (5th edition). Stuttgart:
Georg Thieme Verlag
Thieme Medical Publishers is a German medical and science publisher in the Thieme Publishing Group. It produces professional journals, textbooks, atlases, monographs and reference books in both German and English covering a variety of medical ...
, .
 The
The acyl
In chemistry, an acyl group is a moiety derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids. It contains a double-bonded oxygen atom and an organyl group () or hydrogen in the case of formyl grou ...
compounds required for this reaction can be obtained through a Friedel-Crafts acylation with aluminum chloride
Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula . It forms a hexahydrate with the formula , containing six water molecules of hydration. Both the anhydrous form and the hexahydrate are col ...
.
The Elbs reaction is sometimes accompanied by elimination of substituents and can be unsuited for substituted polyaromatics.
Mechanism
At least three plausible mechanisms for the Elbs reaction have been suggested. The first mechanism, suggested by Fieser, begins with a heat-inducedcyclisation
A cyclic compound (or ring compound) is a term for a chemical compound, compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring (chemistry), ring. Rings may vary in size from three to man ...
of the benzophenone, followed by a ,3hydride shift to give the compound . A dehydration reaction
In chemistry, a dehydration reaction is a chemical reaction that involves the loss of an H2O from the reacting molecule(s) or ion(s). This reaction results in the release of the H2O as water. When the reaction involves the coupling of two molecu ...
then affords the polyaromatic.
tautomerization
In chemistry, tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert.
The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the reloca ...
followed by an electrocyclic reaction
In organic chemistry, an electrocyclic reaction is a type of pericyclic reaction, pericyclic, rearrangement reaction, rearrangement reaction where the net result is one pi bond being converted into one sigma bond or vice versa. These reactions are ...
to give the same intermediate, which then similarly undergoes a ,3hydride shift and dehydration.
pyrolytic
Pyrolysis is a process involving the separation of covalent bonds in organic matter by thermal decomposition within an inert environment without oxygen. Etymology
The word ''pyrolysis'' is coined from the Greek-derived elements ''pyro-'' ( ...
radical generation.
Variations
It is also possible to synthesiseheterocyclic compound
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, proper ...
s via the Elbs reaction. In 1956 an Elbs reaction of a thiophene
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reacti ...
derivative was published. The expected linear product was not obtained due to a change in reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical reaction occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage ...
after formation of the first intermediate which caused multiple free radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabolic disorders
Metabolism
...
reaction steps.G. M. Badger, B. J. Christie. (1956). "Polynuclear heterocyclic systems. Part X. The elbs reaction with heterocyclic ketones." ''J. Chem. Soc.
The ''Journal of the Chemical Society'' was a scientific journal established by the Chemical Society in 1849 as the ''Quarterly Journal of the Chemical Society''. The first editor was Edmund Ronalds. The journal underwent several renamings, splits ...
'' 1956: 3435–3437, {{doi, 10.1039/JR9560003435.

References