Distannene on:
[Wikipedia]
[Google]
[Amazon]
Organotin chemistry is the scientific study of the synthesis and properties of organotin compounds or stannanes, which are
SnRR'R''R have been resolved into individual enantiomers.
File:R2SnO-cyclic-trimer-2D.png, Idealized structure of trimeric diorganotin oxide.
File:TBu2SnO-cyclic-trimer-from-xtal-1984-Mercury-3D-balls.png, Ball-and-stick model for (.
File:R2SnO-cross-linked-network-Harris-and-Sebald-1987-2D.png, Structure of diorganotin oxide, highlighting the extensive intermolecular bonding.
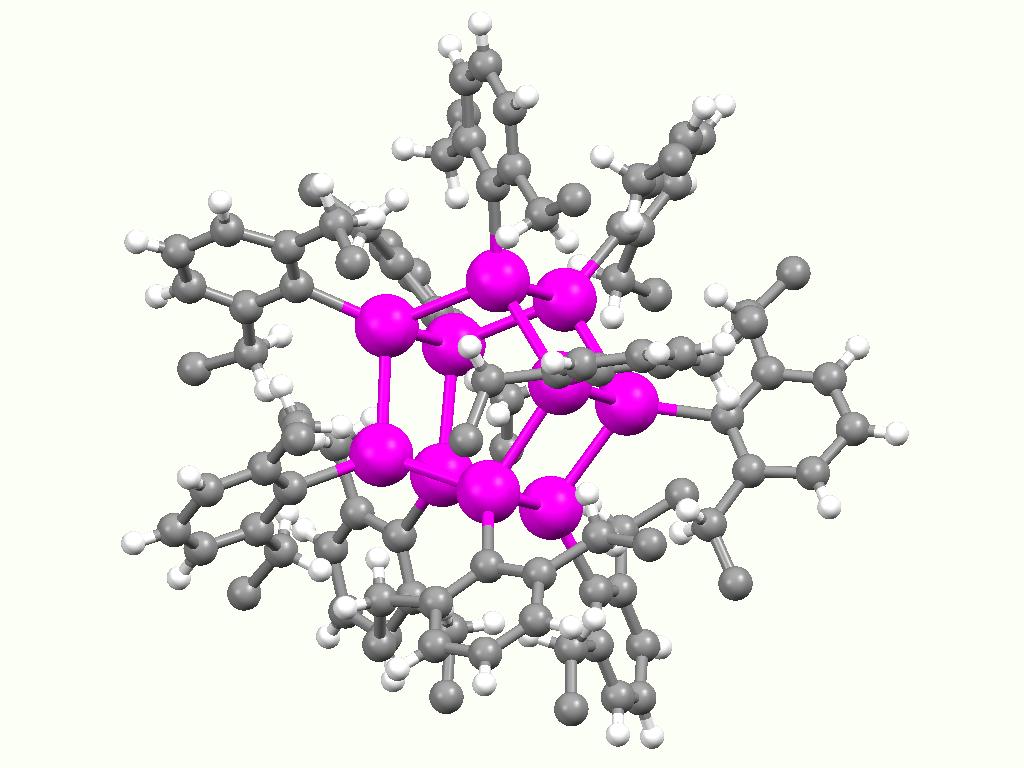
Image:Tetrabutyltin.svg,
National Pollutant Inventory Fact Sheet for organotins
Industry information site
{{ChemicalBondsToCarbon Endocrine disruptors
organometallic compounds
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, an ...
containing tin
Tin is a chemical element; it has symbol Sn () and atomic number 50. A silvery-colored metal, tin is soft enough to be cut with little force, and a bar of tin can be bent by hand with little effort. When bent, a bar of tin makes a sound, the ...
–carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
bonds. The first organotin compound was diethyltin diiodide (), discovered by Edward Frankland
Sir Edward Frankland, (18 January 18259 August 1899) was an English chemist. He was one of the originators of organometallic chemistry and introduced the concept of combining power or valence. An expert in water quality and analysis, he was ...
in 1849. The area grew rapidly in the 1900s, especially after the discovery of the Grignard reagent
Grignard reagents or Grignard compounds are chemical compounds with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromi ...
s, which are useful for producing Sn–C bonds. The area remains rich with many applications in industry and continuing activity in the research laboratory.
Structure
Organotin compounds are generally classified according to their oxidation states. Tin(IV) compounds are much more common and more useful.Organic derivatives of tin(IV)
The tetraorgano derivatives are invariably tetrahedral. Compounds of the typeOrganotin halides
Organotin chlorides have the formula for values of ''n'' up to 3. Bromides, iodides, and fluorides are also known, but are less important. These compounds are known for many R groups. They are always tetrahedral. The tri- and dihalides form adducts with good Lewis bases such aspyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom . It is a highly flammable, weak ...
. The fluorides tend to associate such that dimethyltin difluoride forms sheet-like polymers. Di- and especially tri-organotin halides, e.g. tributyltin chloride
Tributyltin chloride is an organotin compound with the formula ( C4H9)3SnCl. It is a colorless liquid that is soluble in organic solvents.
Preparation and reactions
The compound is prepared by a redistribution reaction by combining stannic chlo ...
, exhibit toxicities approaching that of hydrogen cyanide
Hydrogen cyanide (formerly known as prussic acid) is a chemical compound with the chemical formula, formula HCN and structural formula . It is a highly toxic and flammable liquid that boiling, boils slightly above room temperature, at . HCN is ...
.
Organotin hydrides
Organotin hydrides have the formula for values of ''n'' up to 3. The parent member of this series,stannane
Stannane or tin hydride is an inorganic compound with the chemical formula Sn H4. It is a colourless gas and the tin analogue of methane. Stannane can be prepared by the reaction of and .
:
Stannane decomposes slowly at room temperature to ...
(), is an unstable colourless gas. Stability is correlated with the number of organic substituents. Tributyltin hydride
Tributyltin hydride is an organotin compound with the formula (C4H9)3SnH. It is a colorless liquid that is soluble in organic solvents. The compound is used as a source of hydrogen atoms in organic synthesis.
Synthesis and characterization
The c ...
is used as a source of hydride radical in some organic reactions.
Organotin oxides and hydroxides
Organotin oxides and hydroxides are common products from the hydrolysis of organotin halides. Unlike the corresponding derivatives of silicon and germanium, tin oxides and hydroxides often adopt structures with penta- and even hexacoordinated tin centres, especially for the diorgano- and monoorgano derivatives. The group is called a stannoxane (which is a tin analogue ofethers
In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group (e.g., alkyl or aryl). They have the general formula , where R and R′ r ...
), and the group is also called a stannanol (which is a tin analogue of alcohols
In chemistry, an alcohol (), is a type of organic compound that carries at least one hydroxyl () functional group bound to a Saturated and unsaturated compounds, saturated carbon atom. Alcohols range from the simple, like methanol and ethanol ...
). Structurally simplest of the oxides and hydroxides are the triorganotin derivatives. A commercially important triorganotin hydroxide is the acaricide
Acaricides are pesticides that kill members of the arachnid subclass '' Acari'', which includes ticks and mites.
Acaricides are used both in medicine and agriculture, although the desired selective toxicity differs between the two fields.
Termi ...
cyhexatin
Cyhexatin, also known as tricyclohexyltin hydroxide is an organometallic compound of tin with the chemical formula .
Properties
Cyhexatin forms colorless crystals or white crystalline powder. It is practically insoluble in water. The powder is ...
(also called Plictran, tricyclohexyltin hydroxide and tricyclohexylstannanol), (. Such triorganotin hydroxides exist in equilibrium with the distannoxanes:
:
With only two organic substituents on each Sn centre, the diorganotin oxides and hydroxides are structurally more complex than the triorgano derivatives. The simple tin geminal diols (, the tin analogues of geminal diols
In chemistry, the Descriptor (Chemistry), descriptor geminal () refers to the relationship between two atoms or functional groups that are attached to the same atom. A geminal diol, for example, is a diol (a molecule that has two Alcohol (chemis ...
) and monomeric stannanones (, the tin analogues of ketones
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
) are unknown. Diorganotin oxides () are polymers except when the organic substituents are very bulky, in which case cyclic trimers or, in the case where R is dimers, with and rings. The distannoxanes exist as dimers with the formula wherein the X groups (e.g., chloride
The term chloride refers to a compound or molecule that contains either a chlorine anion (), which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond (). The pr ...
–Cl, hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It ...
–OH, carboxylate
In organic chemistry, a carboxylate is the conjugate base of a carboxylic acid, (or ). It is an anion, an ion with negative charge.
Carboxylate salts are salts that have the general formula , where M is a metal and ''n'' is 1, 2,... ...
) can be terminal or bridging (see Table). The hydrolysis of the monoorganotin trihalides has the potential to generate stannanoic acids, . As for the diorganotin oxides/hydroxides, the monoorganotin species form structurally complex because of the occurrence of dehydration/hydration, aggregation. Illustrative is the hydrolysis of butyltin trichloride to give .
Hypercoordinated stannanes
Unlike carbon(IV) analogues but somewhat like silicon compounds, tin(IV) can also be coordinated to five and even six atoms instead of the regular four. These hypercoordinated compounds usually haveelectronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
substituents. Numerous examples of hypercoordinated compounds are provided by the organotin oxides and associated carboxylates and related pseudohalide derivatives. The organotin halides for adducts, e.g. ).
The all-organic penta- and hexaorganostannates(IV) have even been characterized, while in the subsequent year a six-coordinated tetraorganotin compound was reported. A crystal structure of room-temperature stable (in argon
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
) all-carbon pentaorganostannate(IV) was reported as the lithium
Lithium (from , , ) is a chemical element; it has chemical symbol, symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard temperature and pressure, standard conditions, it is the least dense metal and the ...
salt with this structure:
:
In this distorted trigonal bipyramidal structure the carbon to tin bond length
In molecular geometry, bond length or bond distance is defined as the average distance between Atomic nucleus, nuclei of two chemical bond, bonded atoms in a molecule. It is a Transferability (chemistry), transferable property of a bond between at ...
s (2.26 Å apical
Apical means "pertaining to an apex". It may refer to:
*Apical ancestor, refers to the last common ancestor of an entire group, such as a species (biology) or a clan (anthropology)
*Apical (anatomy), an anatomical term of location for features loc ...
, 2.17 Å equatorial) are longer than regular C-Sn bonds (2.14 Å) reflecting its hypercoordinated nature.
Triorganotin cations
Some reactions of triorganotin halides implicate a role for intermediates. Such cations are analogous tocarbocation
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further ...
s. They have been characterized crystallographically when the organic substituents are large, such as 2,4,6-triisopropylphenyl.
Tin radicals (organic derivatives of tin(III))
Tin radicals, with the formula , are called stannyl radicals.Davies, Alwyn George. (2004) Organotin Chemistry, 2nd Edition Weinheim: Wiley-VCH. They are a type of tetrel radical, and are invoked as intermediates in certain atom-transfer reactions. For example,tributyltin hydride
Tributyltin hydride is an organotin compound with the formula (C4H9)3SnH. It is a colorless liquid that is soluble in organic solvents. The compound is used as a source of hydrogen atoms in organic synthesis.
Synthesis and characterization
The c ...
(tris(''n''-butyl)stannane) serves as a useful source of "hydrogen atoms" because of the stability of the tributytin radical.
Organic derivatives of tin(II)
Organotin(II) compounds are somewhat rare. Compounds with the empirical formula are somewhat fragile and exist as rings or polymers when R is not bulky. The polymers, calledpolystannane
Polystannanes are organotin compounds with the formula (R2Sn)n. These polymers have been of intermittent academic interest; they are unusual because heavy elements comprise the backbone. Structurally related but better characterized (and more us ...
s, have the formula .
:
In principle, compounds of tin(II) might be expected to form a tin analogues of alkenes
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
with a formal double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
between two tin atoms () or between a tin atom and a carbon group
The carbon group is a group (periodic table), periodic table group consisting of carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb), and flerovium (Fl). It lies within the p-block.
In modern International Union of Pure and Applied Ch ...
atom (e.g. and ). Indeed, compounds with the formula , called distannenes or distannylenes, which are tin analogues of ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon–carbon bond, carbon–carbon doub ...
s , are known for certain organic substituents. The Sn centres in stannenes are trigonal. But, contrary to the C centres in alkenes which are trigonal planar
In chemistry, trigonal planar is a molecular geometry model with one atom at the center and three atoms at the corners of an equilateral triangle, called peripheral atoms, all in one plane. In an ideal trigonal planar species, all three ligands a ...
, the Sn centres in stannenes tend to be highly pyramidal
A pyramid () is a structure whose visible surfaces are triangular in broad outline and converge toward the top, making the appearance roughly a pyramid in the geometric sense. The base of a pyramid can be of any polygon shape, such as triangu ...
. Monomeric
A monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called polymerization.
Classification
Chemis ...
compounds with the formula , tin analogues of carbenes
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" may ...
are also known in a few cases. One example is , where R is the very bulky . Such species reversibly dimerize
In chemistry, dimerization is the process of joining two identical or similar Molecular entity, molecular entities by Chemical bond, bonds. The resulting bonds can be either strong or weak. Many symmetrical chemical species are described as dim ...
to the distannylene upon crystallization:
:
Stannenes, compounds with tin-carbon double bonds, are exemplified by derivatives of stannabenzene. Stannole
Stannole is an organotin compound with the chemical formula, formula (carbon, Chydrogen, H)4tin, SnH2. It is classified as a metallole, i.e. an unsaturated five-membered ring containing a heteroatom. It is a structural analog of cyclopentadiene, ...
s, structural analog
A structural analog, also known as a chemical analog or simply an analog, is a chemical compound, compound having a chemical structure, structure similar to that of another compound, but differing from it in respect to a certain component.
It can ...
s of cyclopentadiene
Cyclopentadiene is an organic compound with the chemical formula, formula C5H6. It is often abbreviated CpH because the cyclopentadienyl anion is abbreviated Cp−.
This colorless liquid has a strong and unpleasant odor. At room temperature, ...
, exhibit little C-Sn double bond character.
Organic derivatives of tin(I)
Compounds of Sn(I) are rare and only observed with very bulky ligands. One prominent family of cages is accessed by pyrolysis of the 2,6-diethylphenyl-substituted tristannylene n(C6H3-2,6-Et2)2sub>3, which affords thecubane-type cluster
A cubane-type cluster is an arrangement of atoms in a molecular structure that forms a cube. In the simplest case, the eight vertices are symmetry equivalent and the species has Oh symmetry group, symmetry. Such structure occurs in the hydrocarbon ...
and a prismane
Prismane or Ladenburg benzene is a polycyclic hydrocarbon with the formula C6H6. It is an isomer of benzene, specifically a valence isomer. Prismane is far less stable than benzene. The carbon (and hydrogen) atoms of the prismane molecule are ar ...
. These cages contain Sn(I) and have the formula n(C6H3-2,6-Et2)sub>''n'' where ''n'' = 8, 10 and Et stands for ethyl group
In organic chemistry, an ethyl group (abbr. Et) is an alkyl substituent with the formula , derived from ethane (). ''Ethyl'' is used in the International Union of Pure and Applied Chemistry
The International Union of Pure and Applied ...
. A stannyne contains a tin atom to carbon group atom triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six Electron pair bond, bonding electrons instead of the usual two in a covalent bond, covalent single bond. Triple bonds are stronger than the equivalent covalent bond, sin ...
(e.g. and ), and a distannyne a triple bond between two tin atoms (). Distannynes only exist for extremely bulky substituents. Unlike alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
s, the core of these distannynes are nonlinear, although they are planar. The Sn-Sn distance is 3.066(1) Å, and the Sn-Sn-C angles are 99.25(14)°. Such compounds are prepared by reduction of bulky aryltin(II) halides.

Preparation
Organotin compounds can be synthesised by numerous methods. Classic is the reaction of aGrignard reagent
Grignard reagents or Grignard compounds are chemical compounds with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromi ...
with tin halides for example tin tetrachloride. An example is provided by the synthesis of tetraethyltin:
:
The symmetrical tetraorganotin compounds, especially tetraalkyl derivatives, can then be converted to various mixed chlorides by redistribution reaction
In chemistry, redistribution usually refers to the exchange of anionic ligands bonded to metal and metalloid centers. The conversion does not involve redox
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical ...
s (also known as the "Kocheshkov comproportionation" in the case of organotin compounds):
:
:
:
A related method involves redistribution of tin halides with organoaluminium compound
Organoaluminium chemistry is the study of compounds containing bonds between carbon and aluminium. It is one of the major themes within organometallic chemistry. Illustrative organoaluminium compounds are the dimer trimethylaluminium, the monomer ...
s.
In principle, alkyltin halides can be formed from direct insertion of the metal into the carbon-halogen bond. However, such reactions are temperamental, typically requiring a very weak carbon-halogen bond (e.g. an alkyl iodide
An iodide ion is I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine deficiency ...
or an allyl
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated a ...
halide) or crown-complexed alkali metal salt catalyst. Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any ...
s or an ionic solvent may also promote the reaction.
The mixed organo-halo tin compounds can be converted to the mixed organic derivatives, as illustrated by the synthesis of dibutyldivinyltin:
:
The organotin hydrides are generated by reduction of the mixed alkyl chlorides. For example, treatment of dibutyltin dichloride with lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula or . It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthe ...
gives the dibutyltin dihydride, a colourless distillable oil:
:
The Wurtz-like coupling of alkyl sodium compounds with tin halides yields tetraorganotin compounds.
Hydrostannylation In chemistry, hydrostannylation is the insertion of unsaturated substrates into an Sn-H bond. The reaction occurs under free-radical conditions, but the stereochemistry and regiochemistry are often complex. The reaction gained synthetic importance ...
involves the metal-catalyzed addition of tin hydrides across unsaturated substrates.
Alternatively, stannide
A stannide can refer to an intermetallic compound containing tin combined with one or more other metals; an anion consisting solely of tin atoms or a compound containing such an anion, or, in the field of organometallic chemistry an ionic compound ...
s attack organic electrophiles to give organostannanes, e.g.:
:LiSnMe3 + CCl4 → C(SnMe3)4 + LiCl.
Reactions
Important reactions, discussed above, usually focus on organotinhalides
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluo ...
and pseudohalide
Pseudohalogens are polyatomic analogues of halogens, whose chemistry, resembling that of the true halogens, allows them to substitute for halogens in several classes of chemical compounds. Pseudohalogens occur in pseudohalogen molecules, inorgani ...
s with nucleophiles
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
. All-alkyl organotin compounds generally do not hydrolyze
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysis ...
except in concentrated acid
An acid is a molecule or ion capable of either donating a proton (i.e. Hydron, hydrogen cation, H+), known as a Brønsted–Lowry acid–base theory, Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis ...
; the major exception being tin acetylides. An organostannane addition Organostannane addition is reaction involving the nucleophilic addition of an allyl-, allenyl-, or propargyl- stannane to an aldehyde, imine, or (in rare cases) a ketone. This reaction is widely used for carbonyl allylation.
The addition of an ...
is nucleophilic addition
In organic chemistry, a nucleophilic addition (AN) reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic addit ...
of an allyl
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated a ...
-, allenyl-, or propargyl
In organic chemistry, the propargyl group is a functional group of 2- propynyl with the structure . It is an alkyl group derived from propyne ().
The term propargylic refers to a saturated position ( ''sp''3-hybridized) on a molecular framework ...
stannanes to aldehydes
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
and imines
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bo ...
, whereas hydrostannylation In chemistry, hydrostannylation is the insertion of unsaturated substrates into an Sn-H bond. The reaction occurs under free-radical conditions, but the stereochemistry and regiochemistry are often complex. The reaction gained synthetic importance ...
conveniently reduces only unpolarized multiple bonds.
Organotin hydrides are unstable to strong base, disproportionating to hydrogen gas
Hydrogen is a chemical element; it has symbol H and atomic number 1. It is the lightest and most abundant chemical element in the universe, constituting about 75% of all normal matter. Under standard conditions, hydrogen is a gas of diatomi ...
and distannanes. The latter equilibrate with the corresponding radicals only in the continued presence of base, or if strongly sterically hindered. Conversely, mineral acids cleave distannanes to the organotin halide and more hydrogen gas.
In "pure" organic synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ...
, the Stille reaction
The Stille reaction is a chemical reaction widely used in organic synthesis. The reaction involves the coupling of two organic groups, one of which is carried as an organotin chemistry, organotin compound (also known as organostannanes). A variet ...
is considered is a key coupling technique. In the Stille reaction, sp2-hybridized organic halides
Halocarbon compounds are chemical compounds in which one or more carbon atoms are linked by covalent bonds with one or more halogen atoms (fluorine, chlorine, bromine or iodine – ) resulting in the formation of organofluorine compounds, orga ...
(e.g. vinyl chloride
Vinyl chloride is an organochloride with the formula H2C =CHCl. It is also called vinyl chloride monomer (VCM) or chloroethene. It is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride (PVC). Vinyl chloride is a ...
) catalyzed by palladium
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), ...
:
:
Organotin compounds are also used extensively in radical chemistry (e.g. radical cyclizations, Barton–McCombie deoxygenation
The Barton–McCombie deoxygenation is an organic reaction in which a Hydroxyl, hydroxy functional group in an organic compound is replaced by a hydrogen to give an alkyl group. It is named after British chemists Sir Derek Harold Richard Barton an ...
, Barton decarboxylation
The Barton decarboxylation is a radical reaction in which a carboxylic acid is converted to a thiohydroxamate ester (commonly referred to as a Barton ester). The product is then heated in the presence of a radical initiator and a suitable hydrogen ...
, etc.).
Applications
An organotin compound is commercially applied as stabilizers inpolyvinyl chloride
Polyvinyl chloride (alternatively: poly(vinyl chloride), colloquial: vinyl or polyvinyl; abbreviated: PVC) is the world's third-most widely produced synthetic polymer of plastic (after polyethylene and polypropylene). About 40 million tons of ...
. In this capacity, they suppress degradation by removing allylic chloride groups and by absorbing hydrogen chloride
The Chemical compound, compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hyd ...
. This application consumes about 20,000 tons of tin each year. The main class of organotin compounds are diorganotin dithiolates with the formula . The Sn-S bond is the reactive component. Diorganotin carboxylates, e.g., dibutyltin dilaurate, are used as catalysts for the formation of polyurethanes
Polyurethane (; often abbreviated PUR and PU) is a class of polymers composed of organic chemistry, organic units joined by carbamate (urethane) links. In contrast to other common polymers such as polyethylene and polystyrene, polyurethane term ...
, for vulcanization
Vulcanization (British English: vulcanisation) is a range of processes for hardening rubbers. The term originally referred exclusively to the treatment of natural rubber with sulfur, which remains the most common practice. It has also grown to ...
of silicones
In Organosilicon chemistry, organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane (, where R = Organyl group, organic group). They are typically colorless oils or elastomer, rubber ...
, and transesterification
Transesterification is the process of exchanging the organic functional group R″ of an ester with the organic group R' of an alcohol. These reactions are often catalyzed by the addition of an acid or base catalyst. Strong acids catalyze the r ...
.
''n''-Butyltin trichloride is used in the production of tin dioxide
Tin(IV) oxide, also known as stannic oxide, is the inorganic compound with the formula SnO2. The mineral form of SnO2 is called cassiterite, and this is the main ore of tin. With many other names, this oxide of tin is an important material in ti ...
layers on glass bottles by chemical vapor deposition
Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high-quality, and high-performance, solid materials. The process is often used in the semiconductor industry to produce thin films.
In typical CVD, the wafer (electro ...
.
Biological applications
"Tributyltin
Tributyltin (TBT) is an umbrella term for a class of organotin compounds which contain the group, with a prominent example being tributyltin oxide. For 40 years TBT was used as a biocide in anti-fouling paint, commonly known as bottom paint, ...
s" are used as industrial biocide
A biocide is defined in the European legislation as a chemical substance or microorganism intended to destroy, deter, render harmless, or exert a controlling effect on any harmful organism. The US Environmental Protection Agency (EPA) uses a sli ...
s, e.g. as antifungal agents in textiles and paper, wood pulp and paper mill systems, breweries, and industrial cooling systems. Triphenyltin derivatives are used as active components of antifungal paints and agricultural fungicides. Other triorganotins are used as miticide
Acaricides are pesticides that kill members of the arachnid subclass ''Acari'', which includes ticks and mites.
Acaricides are used both in medicine and agriculture, although the desired selective toxicity differs between the two fields.
Termino ...
s and acaricide
Acaricides are pesticides that kill members of the arachnid subclass '' Acari'', which includes ticks and mites.
Acaricides are used both in medicine and agriculture, although the desired selective toxicity differs between the two fields.
Termi ...
s. Tributyltin oxide
Tributyltin oxide (TBTO) is an organotin compound chiefly used as a biocide (fungicide and molluscicide), especially a wood preservative. Its chemical formula is C4H9)3Snsub>2O. It is a colorless viscous liquid. It is poorly soluble in water (2 ...
has been extensively used as a wood preservative
Wood preservation refers to any method or process, or even technique, used to protect the wood and extend its service life.
Most wood species are susceptible to both biological (''biotic'') and non-biological (''abiotic'') factors that cause d ...
.
Tributyltin compounds were once widely used as marine anti-biofouling
Biofouling or biological fouling is the accumulation of microorganisms, plants, algae, or small animals where it is not wanted on surfaces such as ship and submarine hulls, devices such as water inlets, pipework, grates, ponds, and rivers that ...
agents to improve the efficiency of ocean-going ships. Concerns over toxicity of these compounds (some reports describe biological effects to marine life at a concentration of 1 nanogram
To help compare different ''Order of magnitude, orders of magnitude'', the following lists describe various ''mass'' levels between 10−67 kilogram, kg and 1052 kg. The least massive thing listed here is a graviton, and the most massive thi ...
per liter) led to a worldwide ban by the International Maritime Organization
The International Maritime Organization (IMO; ; ) is a List of specialized agencies of the United Nations, specialized agency of the United Nations responsible for regulating maritime transport. The IMO was established following agreement at a ...
. As anti-fouling compounds, organotin compounds have been replaced by dichlorooctylisothiazolinone
Dichlorooctylisothiazolinone, DCOIT or DCOI, is the organic compound with the formula SC(Cl)=C(Cl)C(O)NC7H15. It is a white solid that melts near room temperature. It is an isothiazolinone, a class of heterocyclic compounds used as biocides. DCO ...
.
Tetrabutyltin
Tetrabutyltin is the organotin compound with the molecular formula or , where Bu is butyl . Sometimes abbreviated TTBT, it is a colorless, lipophilic oil.
Tetrabutyltin is a precursor to tributyltin and dibutyltin compounds. By the redistributi ...
colorless oil, precursor to the other butyl-tin compounds
Image:Tributyltin oxide.png, Tributyltin oxide
Tributyltin oxide (TBTO) is an organotin compound chiefly used as a biocide (fungicide and molluscicide), especially a wood preservative. Its chemical formula is C4H9)3Snsub>2O. It is a colorless viscous liquid. It is poorly soluble in water (2 ...
, a colorless to pale yellow liquid used in wood preservation
Wood preservation refers to any method or process, or even technique, used to protect the wood and extend its service life.
Most wood species are susceptible to both biological (''biotic'') and non-biological (''abiotic'') factors that cause d ...
Image:Fentin acetate.svg, Triphenyltin acetate
Fentin acetate is an organotin compound with the formula (C6H5)3SnO2CCH3. It is a colourless solid that was previously used as a fungicide
Fungicides are pesticides used to kill parasitic fungi or their spores. Fungi can cause serious damage i ...
, an off-white crystalline solid, used as an insecticide
Insecticides are pesticides used to kill insects. They include ovicides and larvicides used against insect eggs and larvae, respectively. The major use of insecticides is in agriculture, but they are also used in home and garden settings, i ...
and a fungicide
Fungicides are pesticides used to kill parasitic fungi or their spores. Fungi can cause serious damage in agriculture, resulting in losses of yield and quality. Fungicides are used both in agriculture and to fight fungal infections in animals, ...
Image:Triphenyltin chloride.png, Triphenyltin chloride
Triphenyltin chloride is an organotin compound with formula Sn(C6H5)3Cl. It is a colourless solid that dissolves in organic solvents and slowly reacts with water. Applications
The main use for this compound is as a fungicide and antifoulant.
Tr ...
, a highly toxic white solid, used as a biocide
Image:Trimethyltin chloride.png, Trimethyltin chloride
Trimethyltin chloride is an organotin compound with the formula . It is a white solid that is highly toxic and malodorous. It is susceptible to hydrolysis.
Synthesis
Trimethyltin chloride can be prepared by the Redistribution (chemistry), redistri ...
, a toxic white solid, once used as a biocide
Image:Triphenyltin hydroxide.svg, Triphenyltin hydroxide
Triphenyltin hydroxide is an organotin compound with formula Sn(C6H5)3OH. Triphenyltin hydroxide is used as a fungicide for potatoes, sugar beets, and pecans. It was first registered for use as a pesticide in the United States in 1971.
Structure
...
, an off-white powder, used as a fungicide
Fungicides are pesticides used to kill parasitic fungi or their spores. Fungi can cause serious damage in agriculture, resulting in losses of yield and quality. Fungicides are used both in agriculture and to fight fungal infections in animals, ...
Image:Azocyclotin.svg, Azocyclotin, a white solid, used as a long-acting acaricide
Acaricides are pesticides that kill members of the arachnid subclass '' Acari'', which includes ticks and mites.
Acaricides are used both in medicine and agriculture, although the desired selective toxicity differs between the two fields.
Termi ...
for control of spider mite
Spider mites are members of the family Tetranychidae, which includes about 1,200 species. They are part of the subclass Acari (mites). Spider mites generally live on the undersides of leaves of plants, where they may spin protective silk webs, a ...
s on plants
Image:Cyhexatin.svg, Cyhexatin
Cyhexatin, also known as tricyclohexyltin hydroxide is an organometallic compound of tin with the chemical formula .
Properties
Cyhexatin forms colorless crystals or white crystalline powder. It is practically insoluble in water. The powder is ...
, a white solid, used as an acaricide
Acaricides are pesticides that kill members of the arachnid subclass '' Acari'', which includes ticks and mites.
Acaricides are used both in medicine and agriculture, although the desired selective toxicity differs between the two fields.
Termi ...
and miticide
Acaricides are pesticides that kill members of the arachnid subclass ''Acari'', which includes ticks and mites.
Acaricides are used both in medicine and agriculture, although the desired selective toxicity differs between the two fields.
Termino ...
Image:Hexamethylditin.svg, Hexamethylditin used as an intermediate in chemical synthesis
Image:Tetraethyltin.svg, Tetraethyltin
Tetraethyltin or tetraethyl tin is a chemical compound with the formula , that is, a tin atom attached to four ethyl groups. It is an important example of an organotin compound, often abbreviated as TET.
Tetraethyltin is a colourless flammable l ...
, boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envi ...
63–65° /12 mm is a catalyst. The "Et" symbol stands for ethyl group
In organic chemistry, an ethyl group (abbr. Et) is an alkyl substituent with the formula , derived from ethane (). ''Ethyl'' is used in the International Union of Pure and Applied Chemistry
The International Union of Pure and Applied ...
.
Toxicity
The toxicities of tributyltin and triphenyltin derivative compounds are comparable to that ofhydrogen cyanide
Hydrogen cyanide (formerly known as prussic acid) is a chemical compound with the chemical formula, formula HCN and structural formula . It is a highly toxic and flammable liquid that boiling, boils slightly above room temperature, at . HCN is ...
. Furthermore, tri-''n''-alkyltins are phytotoxic
Phytotoxins are substances that are poisonous or toxic to the growth of plants. Phytotoxic substances may result from human activity, as with herbicides, or they may be produced by plants, by microorganisms, or by naturally occurring chemical react ...
and therefore cannot be used in agriculture. Depending on the organic groups, they can be powerful bactericide
A bactericide or bacteriocide, sometimes abbreviated Bcidal, is a substance which kills bacteria. Bactericides are disinfectants, antiseptics, or antibiotics.
However, material surfaces can also have bactericidal properties based solely on their p ...
s and fungicide
Fungicides are pesticides used to kill parasitic fungi or their spores. Fungi can cause serious damage in agriculture, resulting in losses of yield and quality. Fungicides are used both in agriculture and to fight fungal infections in animals, ...
s. Reflecting their high bioactivity, "tributyltins" were once used in marine anti-fouling paint
Anti-fouling paint is a specialized category of coatings applied as the outer (outboard) layer to the hull of a ship or boat, to slow the growth of and facilitate detachment of subaquatic organisms that attach to the hull and can affect a ve ...
.
In contrast to the triorganotin compounds, monoorgano, diorgano- and tetraorganotin compounds are far less dangerous, although DBT may be immunotoxic.
See also
*Organostannane addition Organostannane addition is reaction involving the nucleophilic addition of an allyl-, allenyl-, or propargyl- stannane to an aldehyde, imine, or (in rare cases) a ketone. This reaction is widely used for carbonyl allylation.
The addition of an ...
* Tributyltin azide
Tributyltin azide is an organotin compound with the formula (C4H9)3SnN3. It is a colorless solid although samples can appear as yellow oils. The compound is used as a reagent in organic synthesis.
Synthesis and reactions
Tributyltin azide is syn ...
* Carbastannatranes
References
External links
National Pollutant Inventory Fact Sheet for organotins
Industry information site
{{ChemicalBondsToCarbon Endocrine disruptors