Disjoining Pressure on:
[Wikipedia]
[Google]
[Amazon]
In
(Google books)
/ref> : where (in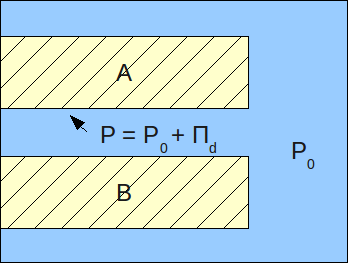 Using the concept of the disjoining pressure, the pressure in a film can be viewed as:
:
where:
* - pressure in a film (Pa)
* - pressure in the bulk of the same phase as that of the film (Pa)
Disjoining pressure is interpreted as a sum of several interactions: dispersion forces, electrostatic forces between charged surfaces, interactions due to layers of neutral molecules adsorbed on the two surfaces, and the structural effects of the solvent.
Classic theory predicts that the disjoining pressure of a thin liquid film on a flat surface as follows,Jacob N. Israelachvili,"Intermolecular and Surface Forces", Academic Press, Revised Third edition, 2011, page 267-26
Using the concept of the disjoining pressure, the pressure in a film can be viewed as:
:
where:
* - pressure in a film (Pa)
* - pressure in the bulk of the same phase as that of the film (Pa)
Disjoining pressure is interpreted as a sum of several interactions: dispersion forces, electrostatic forces between charged surfaces, interactions due to layers of neutral molecules adsorbed on the two surfaces, and the structural effects of the solvent.
Classic theory predicts that the disjoining pressure of a thin liquid film on a flat surface as follows,Jacob N. Israelachvili,"Intermolecular and Surface Forces", Academic Press, Revised Third edition, 2011, page 267-26
(Google books)
/ref> : where: * - Hamaker constant (J) * - liquid film thickness (m) For a solid-liquid-vapor system where the solid surface is structured, the disjoining pressure is affected by the solid surface profile, , and the meniscus shape, : where: * - solid-liquid potential (J/m6) The meniscus shape can be by minimization of total system free energy as follows : where: * - total system free energy including surface excess energy and free energy due to solid-liquid interactions (J/m2) * - meniscus shape (m) * - slope of meniscus shape (1) In the theory of liquid drops and films, the disjoining pressure can be shown to be related to the equilibrium liquid-solid
surface chemistry
Surface science is the study of physics, physical and chemistry, chemical phenomena that occur at the interface (chemistry), interface of two phase (matter), phases, including solid–liquid interfaces, solid–gas interfaces, solid–vacuum int ...
, disjoining pressure (symbol ) according to an IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
definition arises from an attractive interaction between two surfaces. For two flat and parallel surfaces, the value of the disjoining pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and eve ...
(i.e., the force
In physics, a force is an influence that can cause an Physical object, object to change its velocity unless counterbalanced by other forces. In mechanics, force makes ideas like 'pushing' or 'pulling' mathematically precise. Because the Magnitu ...
per unit area) can be calculated as the derivative
In mathematics, the derivative is a fundamental tool that quantifies the sensitivity to change of a function's output with respect to its input. The derivative of a function of a single variable at a chosen input value, when it exists, is t ...
of the Gibbs energy
In thermodynamics, the Gibbs free energy (or Gibbs energy as the recommended name; symbol is a thermodynamic potential that can be used to calculate the maximum amount of work, other than pressure–volume work, that may be performed by a ther ...
of interaction per unit area in respect to distance (in the direction normal to that of the interacting surfaces). There is also a related concept of disjoining force, which can be viewed as disjoining pressure times the surface area of the interacting surfaces.
The concept of disjoining pressure was introduced by Derjaguin (1936) as the difference between the pressure in a region of a phase
Phase or phases may refer to:
Science
*State of matter, or phase, one of the distinct forms in which matter can exist
*Phase (matter), a region of space throughout which all physical properties are essentially uniform
*Phase space, a mathematica ...
adjacent to a surface confining it, and the pressure in the bulk of this phase.
Description
Disjoining pressure can be expressed as:Hans-Jürgen Butt, Karlheinz Graf, Michael Kappl,"Physics and chemistry of interfaces", John Wiley & Sons Canada, Ltd., 1 edition, 2003, page 9(Google books)
/ref> : where (in
SI units
The International System of Units, internationally known by the abbreviation SI (from French ), is the modern form of the metric system and the world's most widely used system of measurement. It is the only system of measurement with official st ...
):
* - disjoining pressure (N/m2)
* - the surface area of the interacting surfaces (m2)
* - total Gibbs energy
In thermodynamics, the Gibbs free energy (or Gibbs energy as the recommended name; symbol is a thermodynamic potential that can be used to calculate the maximum amount of work, other than pressure–volume work, that may be performed by a ther ...
of the interaction of the two surfaces (J)
* - distance (m)
* indices and signify that the temperature, volume, and the surface area remain constant in the derivative.
 Using the concept of the disjoining pressure, the pressure in a film can be viewed as:
:
where:
* - pressure in a film (Pa)
* - pressure in the bulk of the same phase as that of the film (Pa)
Disjoining pressure is interpreted as a sum of several interactions: dispersion forces, electrostatic forces between charged surfaces, interactions due to layers of neutral molecules adsorbed on the two surfaces, and the structural effects of the solvent.
Classic theory predicts that the disjoining pressure of a thin liquid film on a flat surface as follows,Jacob N. Israelachvili,"Intermolecular and Surface Forces", Academic Press, Revised Third edition, 2011, page 267-26
Using the concept of the disjoining pressure, the pressure in a film can be viewed as:
:
where:
* - pressure in a film (Pa)
* - pressure in the bulk of the same phase as that of the film (Pa)
Disjoining pressure is interpreted as a sum of several interactions: dispersion forces, electrostatic forces between charged surfaces, interactions due to layers of neutral molecules adsorbed on the two surfaces, and the structural effects of the solvent.
Classic theory predicts that the disjoining pressure of a thin liquid film on a flat surface as follows,Jacob N. Israelachvili,"Intermolecular and Surface Forces", Academic Press, Revised Third edition, 2011, page 267-26(Google books)
/ref> : where: * - Hamaker constant (J) * - liquid film thickness (m) For a solid-liquid-vapor system where the solid surface is structured, the disjoining pressure is affected by the solid surface profile, , and the meniscus shape, : where: * - solid-liquid potential (J/m6) The meniscus shape can be by minimization of total system free energy as follows : where: * - total system free energy including surface excess energy and free energy due to solid-liquid interactions (J/m2) * - meniscus shape (m) * - slope of meniscus shape (1) In the theory of liquid drops and films, the disjoining pressure can be shown to be related to the equilibrium liquid-solid
contact angle
The contact angle (symbol ) is the angle between a liquid surface and a solid surface where they meet. More specifically, it is the angle between the surface tangent on the liquid–vapor interface and the tangent on the solid–liquid interfac ...
through the relation
:
where is the liquid-vapor surface tension
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension (physics), tension is what allows objects with a higher density than water such as razor blades and insects (e.g. Ge ...
and is the precursor film thickness.
See also
* Capillary condensation *Capillary pressure
In fluid statics, capillary pressure () is the pressure between two immiscible fluids in a thin tube (see capillary action), resulting from the interactions of forces between the fluids and solid walls of the tube. Capillary pressure can serve as b ...
* Hamaker constant
*Thin-film equation
In fluid mechanics, the thin-film equation is a partial differential equation that approximately predicts the time evolution of the thickness of a liquid film that lies on a surface. The equation is derived via lubrication theory which is based o ...
References