Directing Group on:
[Wikipedia]
[Google]
[Amazon]
In  The Murai reaction is related to
The Murai reaction is related to
Abstract
/ref>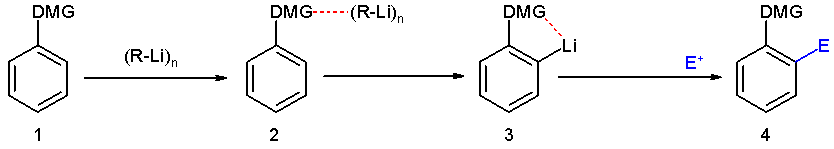 A wide variety of
A wide variety of

organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clay ...
, a directing group (DG) is a substituent
A substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule. (In organic chemistry and biochemistry, the terms ''substituent'' and '' functional group'', as well as '' ...
on a molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bio ...
or ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
that facilitates reactions
Reaction may refer to a process or to a response to an action, event, or exposure:
Physics and chemistry
*Chemical reaction
*Nuclear reaction
*Reaction (physics), as defined by Newton's third law
* Chain reaction (disambiguation).
Biology and me ...
by interacting with a reagent. The term is usually applied to C-H activation of hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
s, where it is defined as a "coordinating moiety
Moiety may refer to:
Chemistry
* Moiety (chemistry), a part or functional group of a molecule
** Moiety conservation, conservation of a subgroup in a chemical species
Anthropology
* Moiety (kinship), either of two groups into which a society is ...
(an “internal ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's ele ...
”), which directs a metal catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
into the proximity of a certain C–H bond." In a well known example, the ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bon ...
group () in acetophenone
Acetophenone is the organic compound with the formula C6H5C(O)CH3. It is the simplest aromatic ketone. This colorless, viscous liquid is a precursor to useful resins and fragrances.
Production
Acetophenone is formed as a byproduct of the cumene ...
is the DG in the Murai reaction.
 The Murai reaction is related to
The Murai reaction is related to directed ortho metalation
Directed ortho metalation (DoM) is an adaptation of electrophilic aromatic substitution in which electrophiles attach themselves exclusively to the ortho- position of a direct metalation group or DMG through the intermediary of an aryllithium co ...
, a reaction is typically applied to the lithiation
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
of substituted aromatic rings.''Directed ortho metalation. Tertiary amide and O-carbamate directors in synthetic strategies for polysubstituted aromatics Victor Snieckus'' Chem. Rev.
''Chemical Reviews'' is peer-reviewed scientific journal published twice per month by the American Chemical Society. It publishes review articles on all aspects of chemistry. It was established in 1924 by William Albert Noyes (University of Illino ...
; 1990; 90(6); 879-933Abstract
/ref>
 A wide variety of
A wide variety of functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the res ...
s can serve as directing groups.
Transient directing groups
Since directing groups are ligands, their effectiveness correlates with their affinities for metals. Common functional groups such as ketones usually are only weak ligands and thus often are poor DGs. This problem is solved by the use of a transient directing group. Transient DGs reversibly convert weak DGs (e.g., ketones) into strong DG's (e.g., imines) via aSchiff base
In organic chemistry, a Schiff base (named after Hugo Schiff) is a compound with the general structure ( = alkyl or aryl, but not hydrogen). They can be considered a sub-class of imines, being either secondary ketimines or secondary aldimine ...
condensation. Subsequent to serving their role as DGs, the imine can hydrolyze, regenerating the ketone and amine.{{cite journal, authors=St John-Campbell, S. and J. A. Bull, year=2018, title=Transient imines as 'next generation' directing groups for the catalytic functionalisation of C–H bonds in a single operation, journal=Organic & Biomolecular Chemistry, volume=16, issue=25, pages= 4582–4595, doi=10.1039/C8OB00926K, pmid=29796566, url=http://spiral.imperial.ac.uk/bitstream/10044/1/60231/2/MS%20transient%20review%20OBC%20revised.pdf, hdl=10044/1/60231, hdl-access=free

References