
Diamond is a
solid form of the element carbon with its atoms arranged in a
crystal structure
In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that repeat ...
called
diamond cubic
In crystallography, the diamond cubic crystal structure is a repeating pattern of 8 atoms that certain materials may adopt as they solidify. While the first known example was diamond, other elements in group 14 also adopt this structure, in ...
. Diamond is tasteless, odourless, strong, brittle solid, colourless in pure form, a poor conductor of electricity, and insoluble in water. Another solid form of carbon known as
graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable ...
is the
chemically stable form of carbon at
room temperature and pressure, but diamond is
metastable
In chemistry and physics, metastability is an intermediate energetic state within a dynamical system other than the system's state of least energy.
A ball resting in a hollow on a slope is a simple example of metastability. If the ball is onl ...
and converts to it at a negligible rate under those conditions. Diamond has the highest
hardness
In materials science, hardness (antonym: softness) is a measure of the resistance to plastic deformation, such as an indentation (over an area) or a scratch (linear), induced mechanically either by Pressing (metalworking), pressing or abrasion ...
and
thermal conductivity
The thermal conductivity of a material is a measure of its ability to heat conduction, conduct heat. It is commonly denoted by k, \lambda, or \kappa and is measured in W·m−1·K−1.
Heat transfer occurs at a lower rate in materials of low ...
of any natural material, properties that are used in major industrial applications such as cutting and polishing tools.
Because the arrangement of atoms in diamond is extremely rigid, few types of impurity can contaminate it (two exceptions are
boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three ...
and
nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
). Small numbers of
defects or impurities (about one per million of lattice atoms) can color a diamond blue (boron), yellow (nitrogen), brown (defects), green (radiation exposure), purple, pink, orange, or red. Diamond also has a very high
refractive index
In optics, the refractive index (or refraction index) of an optical medium is the ratio of the apparent speed of light in the air or vacuum to the speed in the medium. The refractive index determines how much the path of light is bent, or refrac ...
and a relatively high
optical dispersion
Dispersion is the phenomenon in which the phase velocity of a wave depends on its frequency. Sometimes the term chromatic dispersion is used to refer to optics specifically, as opposed to wave propagation in general. A medium having this common ...
.
Most natural diamonds have ages between 1 billion and 3.5 billion years. Most were formed at depths between in the Earth's
mantle, although a few have come from as deep as . Under high pressure and temperature, carbon-containing fluids dissolved various minerals and replaced them with diamonds. Much more recently (hundreds to tens of million years ago), they were carried to the surface in
volcanic eruption
A volcanic eruption occurs when material is expelled from a volcanic vent or fissure. Several types of volcanic eruptions have been distinguished by volcanologists. These are often named after famous volcanoes where that type of behavior h ...
s and deposited in
igneous rock
Igneous rock ( ), or magmatic rock, is one of the three main rock types, the others being sedimentary and metamorphic. Igneous rocks are formed through the cooling and solidification of magma or lava.
The magma can be derived from partial ...
s known as
kimberlite
Kimberlite is an igneous rock and a rare variant of peridotite. It is most commonly known as the main host matrix for diamonds. It is named after the town of Kimberley, Northern Cape, Kimberley in South Africa, where the discovery of an 83.5-Car ...
s and
lamproite
Lamproite is an ultrapotassic mantle-derived volcanic or subvolcanic rock. It has low CaO, Al2O3, Na2O, high K2O/Al2O3, a relatively high MgO content and extreme enrichment in incompatible elements.
Lamproites are geographically widespre ...
s.
Synthetic diamond
A synthetic diamond or laboratory-grown diamond (LGD), also called a lab-grown, laboratory-created, man-made, artisan-created, artificial, or cultured diamond, is a diamond that is produced in a controlled technological process, in contrast to ...
s can be grown from high-purity carbon under high pressures and temperatures or from
hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually fain ...
gases by
chemical vapor deposition
Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high-quality, and high-performance, solid materials. The process is often used in the semiconductor industry to produce thin films.
In typical CVD, the wafer (electro ...
(CVD). Natural and synthetic diamonds are most commonly distinguished using optical techniques or thermal conductivity measurements.
Etymology, earliest use and composition discovery
The name ''diamond'' is derived from (''adámas''), 'proper, unalterable, unbreakable, untamed', from
ἀ- (''a-''), 'not' + (''damáō''), 'to overpower, tame'. Diamonds are thought to have been first recognized and mined in
India
India, officially the Republic of India, is a country in South Asia. It is the List of countries and dependencies by area, seventh-largest country by area; the List of countries by population (United Nations), most populous country since ...
, where significant
alluvial deposit
Alluvium (, ) is loose clay, silt, sand, or gravel that has been deposited by running water in a stream bed, on a floodplain, in an alluvial fan or beach, or in similar settings. Alluvium is also sometimes called alluvial deposit. Alluvium is ...
s of the stone could be found many centuries ago along the rivers
Penner,
Krishna
Krishna (; Sanskrit language, Sanskrit: कृष्ण, ) is a major deity in Hinduism. He is worshipped as the eighth avatar of Vishnu and also as the Supreme God (Hinduism), Supreme God in his own right. He is the god of protection, c ...
, and
Godavari
The Godavari (, �od̪aːʋəɾiː is India's second longest river after the Ganga River and drains the third largest basin in India, covering about 10% of India's total geographical area. Its source is in Trimbakeshwar, Nashik, Maharash ...
. Diamonds have been known in India for at least 3,000years but most likely 6,000years.
Diamonds have been treasured as gemstones since their use as
religious icons in
ancient India
Anatomically modern humans first arrived on the Indian subcontinent between 73,000 and 55,000 years ago. The earliest known human remains in South Asia date to 30,000 years ago. Sedentism, Sedentariness began in South Asia around 7000 BCE; ...
. Their usage in engraving tools also dates to early
human history
Human history or world history is the record of humankind from prehistory to the present. Early modern human, Modern humans evolved in Africa around 300,000 years ago and initially lived as hunter-gatherers. They Early expansions of hominin ...
.
The popularity of diamonds has risen since the 19th century because of increased supply, improved cutting and polishing techniques, growth in the world economy, and innovative and successful advertising campaigns.
In 1772, the French scientist
Antoine Lavoisier
Antoine-Laurent de Lavoisier ( ; ; 26 August 17438 May 1794), When reduced without charcoal, it gave off an air which supported respiration and combustion in an enhanced way. He concluded that this was just a pure form of common air and that i ...
used a lens to concentrate the rays of the sun on a diamond in an atmosphere of
oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
, and showed that the only product of the combustion was
carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
, proving that diamond is composed of carbon. Later, in 1797, the English chemist
Smithson Tennant
Smithson Tennant Royal Society#Fellows, FRS (30 November 1761 – 22 February 1815) was an English chemist. He is best known for his discovery of the elements iridium and osmium, which he found in the residues from the solution of platinum ores ...
repeated and expanded that experiment. By demonstrating that burning diamond and graphite releases the same amount of gas, he established the chemical equivalence of these substances.
[
]
Properties
Diamond is a solid form of pure carbon with its atoms arranged in a crystal. Solid carbon comes in different forms known as allotrope
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes are different structural modifications of an element: the ...
s depending on the type of chemical bond. The two most common allotropes of pure carbon are diamond and graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable ...
. In graphite, the bonds are sp2 orbital hybrids and the atoms form in planes, with each bound to three nearest neighbors, 120 degrees apart. In diamond, they are sp3 and the atoms form tetrahedra, with each bound to four nearest neighbors. Tetrahedra are rigid, the bonds are strong, and, of all known substances, diamond has the greatest number of atoms per unit volume, which is why it is both the hardest and the least compressible
In thermodynamics and fluid mechanics, the compressibility (also known as the coefficient of compressibility or, if the temperature is held constant, the isothermal compressibility) is a measure of the instantaneous relative volume change of a f ...
.thermal conductivity
The thermal conductivity of a material is a measure of its ability to heat conduction, conduct heat. It is commonly denoted by k, \lambda, or \kappa and is measured in W·m−1·K−1.
Heat transfer occurs at a lower rate in materials of low ...
and the highest sound velocity. It has low adhesion and friction, and its coefficient of thermal expansion
Thermal expansion is the tendency of matter to increase in length, area, or volume, changing its size and density, in response to an increase in temperature (usually excluding phase transitions).
Substances usually contract with decreasing temp ...
is extremely low. Its optical transparency extends from the far infrared
Far infrared (FIR) or long wave refers to a specific range within the infrared spectrum of electromagnetic radiation. It encompasses radiation with wavelengths ranging from 15 μm ( micrometers) to 1 mm, which corresponds to a freque ...
to the deep ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of ...
and it has high optical dispersion
Dispersion is the phenomenon in which the phase velocity of a wave depends on its frequency. Sometimes the term chromatic dispersion is used to refer to optics specifically, as opposed to wave propagation in general. A medium having this common ...
. It also has high electrical resistance. It is chemically inert, not reacting with most corrosive substances, and has excellent biological compatibility.
Thermodynamics
 The equilibrium pressure and temperature conditions for a transition between graphite and diamond are well established theoretically and experimentally. The equilibrium pressure varies linearly with temperature, between at and at (the diamond/graphite/liquid
The equilibrium pressure and temperature conditions for a transition between graphite and diamond are well established theoretically and experimentally. The equilibrium pressure varies linearly with temperature, between at and at (the diamond/graphite/liquid triple point
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three Phase (matter), phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.. It is that temperature and pressure at ...
).standard temperature and pressure
Standard temperature and pressure (STP) or standard conditions for temperature and pressure are various standard sets of conditions for experimental measurements used to allow comparisons to be made between different sets of data. The most used ...
, and , the stable phase of carbon is graphite, but diamond is metastable
In chemistry and physics, metastability is an intermediate energetic state within a dynamical system other than the system's state of least energy.
A ball resting in a hollow on a slope is a simple example of metastability. If the ball is onl ...
, with a significant kinetic energy barrier that the atoms must overcome in order to reach the lower energy state,[ However, at temperatures above about , diamond rapidly converts to graphite. Experiments have found that diamond in the presence of passes through an intermediate linear carbon phase.
Rapid conversion of graphite to diamond requires pressures well above the equilibrium line: at , a pressure of (about 350,000 standard atmospheres) is needed.][
Above the graphite–diamond–liquid carbon triple point, the melting point of diamond increases slowly with increasing pressure; but at pressures of hundreds of GPa, it decreases. At high pressures, ]silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
and germanium
Germanium is a chemical element; it has Symbol (chemistry), symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid or a nonmetal in the carbon group that is chemically ...
have a BC8 body-centered cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the Crystal structure#Unit cell, unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There ...
crystal structure, and a similar structure is predicted for carbon at high pressures. At , the transition is predicted to occur at .
Results published in ''Nature Physics
''Nature Physics'' is a monthly peer-reviewed scientific journal published by Nature Portfolio. It was first published in October 2005 (volume 1, issue 1). The chief editor is David Abergel.
Scope
''Nature Physics'' publishes both pure and appli ...
'' in 2010 suggest that, at ultra-high pressures and temperatures (about 10 million atmospheres or 1 TPa and 50,000 °C), diamond melts into a metallic fluid. The extreme conditions required for this to occur are present in the ice giant
An ice giant is a giant planet composed mainly of elements heavier than hydrogen and helium, such as oxygen, carbon, nitrogen, and sulfur. There are two ice giants in the Solar System: Uranus and Neptune.
In astrophysics and planetary science ...
planet
A planet is a large, Hydrostatic equilibrium, rounded Astronomical object, astronomical body that is generally required to be in orbit around a star, stellar remnant, or brown dwarf, and is not one itself. The Solar System has eight planets b ...
s Neptune
Neptune is the eighth and farthest known planet from the Sun. It is the List of Solar System objects by size, fourth-largest planet in the Solar System by diameter, the third-most-massive planet, and the densest giant planet. It is 17 t ...
and Uranus
Uranus is the seventh planet from the Sun. It is a gaseous cyan-coloured ice giant. Most of the planet is made of water, ammonia, and methane in a Supercritical fluid, supercritical phase of matter, which astronomy calls "ice" or Volatile ( ...
, both of which are made up of approximately 10 percent carbon and could hypothetically contain oceans of liquid carbon. Since large quantities of metallic fluid can affect the magnetic field, this could serve to explain why the geographic and magnetic poles of the two planets are not aligned.
Crystal structure
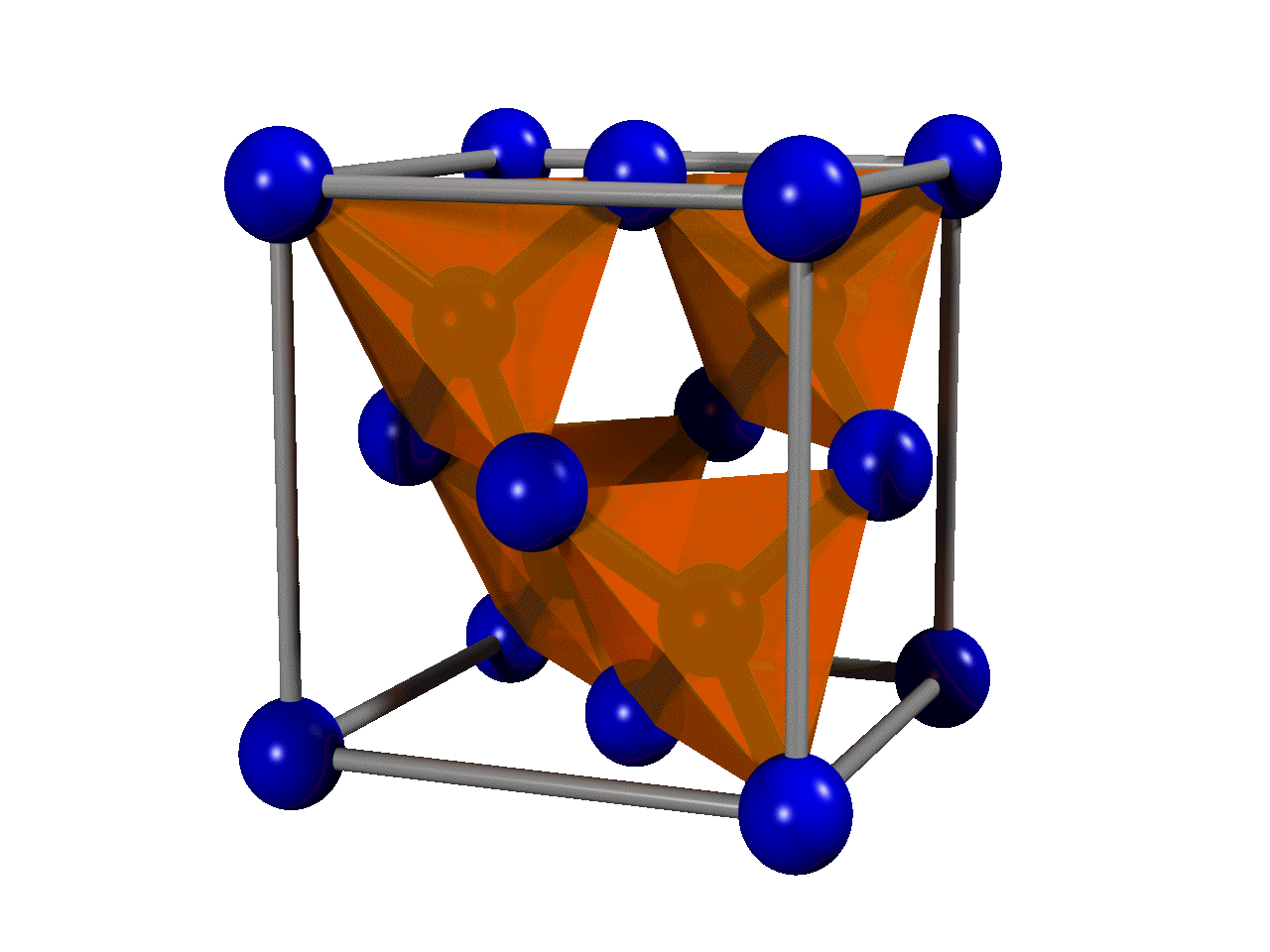 The most common crystal structure of diamond is called
The most common crystal structure of diamond is called diamond cubic
In crystallography, the diamond cubic crystal structure is a repeating pattern of 8 atoms that certain materials may adopt as they solidify. While the first known example was diamond, other elements in group 14 also adopt this structure, in ...
. It is formed of unit cell
In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a unit vector
In mathematics, a unit vector i ...
s (see the figure) stacked together. Although there are 18 atoms in the figure, each corner atom is shared by eight unit cells and each atom in the center of a face is shared by two, so there are a total of eight atoms per unit cell. The length of each side of the unit cell is denoted by ''a'' and is 3.567 angstrom
The angstrom (; ) is a unit of length equal to m; that is, one ten-billionth of a metre, a hundred-millionth of a centimetre, 0.1 nanometre, or 100 picometres. The unit is named after the Swedish physicist Anders Jonas Ångström (1814–18 ...
s.face-centered cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties o ...
lattices with one displaced by of the diagonal along a cubic cell, or as one lattice with two atoms associated with each lattice point.[ Viewed from a crystallographic direction, it is formed of layers stacked in a repeating ABCABC ... pattern. Diamonds can also form an ABAB ... structure, which is known as hexagonal diamond or ]lonsdaleite
Lonsdaleite (named in honour of Kathleen Lonsdale), also called hexagonal diamond in reference to the crystal structure, is an allotrope of carbon with a hexagonal lattice, as opposed to the cubical lattice of conventional diamond. It is found ...
, but this is far less common and is formed under different conditions from cubic carbon.
Crystal habit
 Diamonds occur most often as
Diamonds occur most often as euhedral
Euhedral and anhedral are terms used to describe opposite properties in the formation of crystals. Euhedral (also known as idiomorphic or automorphic) crystals
are those that are well-formed, with sharp, easily recognised faces. The opposite i ...
or rounded octahedra
In geometry, an octahedron (: octahedra or octahedrons) is any polyhedron with eight faces. One special case is the regular octahedron, a Platonic solid composed of eight equilateral triangles, four of which meet at each vertex. Many types of i ...
and twinned octahedra known as ''macle
Macle is a term used in crystallography. It is a crystalline form, twin-crystal or double crystal (such as chiastolite). It is crystallographic twin according to the spinel twin law and is seen in octahedral crystals or minerals such as dia ...
s''. As diamond's crystal structure has a cubic arrangement of the atoms, they have many facet
Facets () are flat faces on geometric shapes. The organization of naturally occurring facets was key to early developments in crystallography, since they reflect the underlying symmetry of the crystal structure. Gemstones commonly have facets cu ...
s that belong to a cube
A cube or regular hexahedron is a three-dimensional space, three-dimensional solid object in geometry, which is bounded by six congruent square (geometry), square faces, a type of polyhedron. It has twelve congruent edges and eight vertices. It i ...
, octahedron, rhombicosidodecahedron
In geometry, the rhombicosidodecahedron is an Archimedean solid, one of thirteen convex isogonal nonprismatic solids constructed of two or more types of regular polygon faces.
It has a total of 62 faces: 20 regular triangular faces, 30 square f ...
, tetrakis hexahedron
In geometry, a tetrakis hexahedron (also known as a tetrahexahedron, hextetrahedron, tetrakis cube, and kiscube) is a Catalan solid. Its dual is the truncated octahedron, an Archimedean solid.
It can be called a disdyakis hexahedron or hexaki ...
, or disdyakis dodecahedron
In geometry, a disdyakis dodecahedron, (also hexoctahedron, hexakis octahedron, octakis cube, octakis hexahedron, kisrhombic dodecahedron) or d48, is a Catalan solid with 48 faces and the dual to the Archimedean truncated cuboctahedron. As such ...
. The crystals can have rounded-off and unexpressive edges and can be elongated. Diamonds (especially those with rounded crystal faces) are commonly found coated in ''nyf'', an opaque gum-like skin.
Some diamonds contain opaque fibers. They are referred to as ''opaque'' if the fibers grow from a clear substrate or ''fibrous'' if they occupy the entire crystal. Their colors range from yellow to green or gray, sometimes with cloud-like white to gray impurities. Their most common shape is cuboidal, but they can also form octahedra, dodecahedra, macles, or combined shapes. The structure is the result of numerous impurities with sizes between 1 and 5 microns. These diamonds probably formed in kimberlite magma and sampled the volatiles.ballas
Ballas (or shot bort) is a diamond industry term for roughly spherical shards of non- gem-grade diamond, mostly mined in Brazil and South Africa
South Africa, officially the Republic of South Africa (RSA), is the Southern Africa, souther ...
, stewartite, and framesite, but there is no widely accepted set of criteria.[ ]Carbonado
Carbonado, commonly known as black diamond, is one of the toughest forms of natural diamond. It is an impure, high-density, micro-porous form of polycrystalline diamond consisting of diamond, graphite, and amorphous carbon, with minor crystall ...
, a type in which the diamond grains were sintered
Sintering or frittage is the process of compacting and forming a solid mass of material by pressure or heat without melting it to the point of liquefaction. Sintering happens as part of a manufacturing process used with metals, ceramics, pla ...
(fused without melting by the application of heat and pressure), is black in color and tougher than single crystal diamond. It has never been observed in a volcanic rock. There are many theories for its origin, including formation in a star, but no consensus.[
]
Mechanical
Hardness
 Diamond is the hardest material on the qualitative
Diamond is the hardest material on the qualitative Mohs scale
The Mohs scale ( ) of mineral hardness is a qualitative ordinal scale, from 1 to 10, characterizing scratch resistance of minerals through the ability of harder material to scratch softer material.
The scale was introduced in 1812 by the Ger ...
. To conduct the quantitative
Quantitative may refer to:
* Quantitative research, scientific investigation of quantitative properties
* Quantitative analysis (disambiguation)
* Quantitative verse, a metrical system in poetry
* Statistics, also known as quantitative analysis
...
Vickers hardness test
The Vickers hardness test was developed in 1921 by Robert L. Smith and George E. Sandland at Vickers Ltd as an alternative to the Brinell scale, Brinell method to measure the hardness of materials. The Vickers test is often easier to use than ot ...
, samples of materials are struck with a pyramid of standardized dimensions using a known force – a diamond crystal is used for the pyramid to permit a wide range of materials to be tested. From the size of the resulting indentation, a Vickers hardness value for the material can be determined. Diamond's great hardness relative to other materials has been known since antiquity, and is the source of its name. This does not mean that it is infinitely hard, indestructible, or unscratchable. Indeed, diamonds can be scratched by other diamonds and worn down over time even by softer materials, such as vinyl phonograph record
A phonograph record (also known as a gramophone record, especially in British English) or a vinyl record (for later varieties only) is an analog sound storage medium in the form of a flat disc with an inscribed, modulated spiral groove. The g ...
s.
Diamond hardness depends on its purity, crystalline perfection, and orientation: hardness is higher for flawless, pure crystals oriented to the <111> direction (along the longest diagonal of the cubic diamond lattice). Therefore, whereas it might be possible to scratch some diamonds with other materials, such as boron nitride
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula B N. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexago ...
, the hardest diamonds can only be scratched by other diamonds and nanocrystalline diamond aggregates.
The hardness of diamond contributes to its suitability as a gemstone. Because it can only be scratched by other diamonds, it maintains its polish extremely well. Unlike many other gems, it is well-suited to daily wear because of its resistance to scratching—perhaps contributing to its popularity as the preferred gem in engagement
An engagement or betrothal is the period of time between the declaration of acceptance of a marriage proposal and the marriage itself (which is typically but not always commenced with a wedding). During this period, a couple is said to be ''f ...
or wedding ring
A wedding ring or wedding band is a finger ring that indicates that its wearer is married. It is usually forged from metal, traditionally gold or another precious metal. Rings were used in ancient Rome during marriage.
In western culture, a ...
s, which are often worn every day.
The hardest natural diamonds mostly originate from the Copeton and Bingara
Bingara (Aboriginal for 'creek') is a small town on the Gwydir River in Murchison County in the New England region of New South Wales, Australia. Bingara is currently the administrative centre for the Gwydir Shire that was created in 2003. T ...
fields located in the New England
New England is a region consisting of six states in the Northeastern United States: Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, and Vermont. It is bordered by the state of New York (state), New York to the west and by the ...
area in New South Wales
New South Wales (commonly abbreviated as NSW) is a States and territories of Australia, state on the Eastern states of Australia, east coast of :Australia. It borders Queensland to the north, Victoria (state), Victoria to the south, and South ...
, Australia. These diamonds are generally small, perfect to semiperfect octahedra, and are used to polish other diamonds. Their hardness is associated with the crystal growth
Crystal growth is a major stage of a crystallization, crystallization process, and consists of the addition of new atoms, ions, or polymer strings into the characteristic arrangement of the crystalline lattice. The growth typically follows an ini ...
form, which is single-stage crystal growth. Most other diamonds show more evidence of multiple growth stages, which produce inclusions, flaws, and defect planes in the crystal lattice, all of which affect their hardness. It is possible to treat regular diamonds under a combination of high pressure and high temperature to produce diamonds that are harder than the diamonds used in hardness gauges.
Diamonds cut glass, but this does not positively identify a diamond because other materials, such as quartz, also lie above glass on the Mohs scale
The Mohs scale ( ) of mineral hardness is a qualitative ordinal scale, from 1 to 10, characterizing scratch resistance of minerals through the ability of harder material to scratch softer material.
The scale was introduced in 1812 by the Ger ...
and can also cut it. Diamonds can scratch other diamonds, but this can result in damage to one or both stones. Hardness tests are infrequently used in practical gemology because of their potentially destructive nature.[ The extreme hardness and high value of diamond means that gems are typically polished slowly, using painstaking traditional techniques and greater attention to detail than is the case with most other gemstones;]diamantaire
A diamantaire (''French origin'') could be a gem-quality diamond manufacturer or producer, master diamond cutter, and or a graduate gemologist specializing in diamonds.
Such individuals demonstrate considerable expertise in different types of g ...
s still rely upon skilled use of a loupe
A loupe ( ) is a simple, small magnification device used to see small details more closely. They generally have higher magnification than a magnifying glass, and are designed to be held or worn close to the eye. A loupe does not have an attached ...
(magnifying glass) to identify diamonds "by eye".
Toughness
Somewhat related to hardness is another mechanical property ''toughness'', which is a material's ability to resist breakage from forceful impact. The toughness
In materials science and metallurgy, toughness is the ability of a material to absorb energy and plastically deform without fracturing.[MPa
MPA or mPa may refer to:
Academia
Academic degrees
* Master of Performing Arts
* Master of Professional Accountancy
* Master of Public Administration
* Master of Public Affairs
Schools
* Mesa Preparatory Academy
* Morgan Park Academy
* M ...]
·m1/2. This value is good compared to other ceramic materials, but poor compared to most engineering materials such as engineering alloys, which typically exhibit toughness over 80MPa·m1/2. As with any material, the macroscopic geometry of a diamond contributes to its resistance to breakage. Diamond has a cleavage plane
Cleavage, in mineralogy and materials science, is the tendency of crystalline materials to split along definite crystallographic structural planes. These planes of relative weakness are a result of the regular locations of atoms and ions in the ...
and is therefore more fragile in some orientations than others. Diamond cutters use this attribute to cleave some stones before faceting them.[ "Impact toughness" is one of the main indexes to measure the quality of synthetic industrial diamonds.
]
Yield strength
Diamond has compressive yield strength of 130–140GPa. This exceptionally high value, along with the hardness and transparency of diamond, are the reasons that diamond anvil
A diamond anvil cell (DAC) is a high-pressure device used in geology, engineering, and materials science experiments. It permits the compression of a small (sub- millimeter-sized) piece of material to extreme pressures, typically up to around 1 ...
cells are the main tool for high pressure experiments.
Elasticity and tensile strength
Usually, attempting to deform bulk diamond crystal by tension or bending results in brittle fracture. However, when single crystalline diamond is in the form of micro/nanoscale wires or needles (~100–300nanometers in diameter, micrometers long), they can be elastically stretched by as much as 9–10 percent tensile strain without failure, with a maximum local tensile stress of about , very close to the theoretical limit for this material.
Electrical conductivity
Other specialized applications also exist or are being developed, including use as semiconductor
A semiconductor is a material with electrical conductivity between that of a conductor and an insulator. Its conductivity can be modified by adding impurities (" doping") to its crystal structure. When two regions with different doping level ...
s: some blue diamond
Blue diamond is a type of diamond which exhibits all of the same inherent properties of the mineral except with the additional element of blue color in the stone. They are colored blue by trace impurities of boron within the crystalline lattice s ...
s are natural semiconductors, in contrast to most diamonds, which are excellent electrical insulators. The conductivity and blue color originate from boron impurity. Boron substitutes for carbon atoms in the diamond lattice, donating a hole into the valence band
In solid-state physics, the valence band and conduction band are the bands closest to the Fermi level, and thus determine the electrical conductivity of the solid. In nonmetals, the valence band is the highest range of electron energies in ...
.chemical vapor deposition
Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high-quality, and high-performance, solid materials. The process is often used in the semiconductor industry to produce thin films.
In typical CVD, the wafer (electro ...
. This conductivity is associated with hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
-related species adsorbed at the surface, and it can be removed by annealing or other surface treatments.
Thin needles of diamond can be made to vary their electronic band gap
In solid-state physics and solid-state chemistry, a band gap, also called a bandgap or energy gap, is an energy range in a solid where no electronic states exist. In graphs of the electronic band structure of solids, the band gap refers to t ...
from the normal 5.6 eV to near zero by selective mechanical deformation.
High-purity diamond wafers 5 cm in diameter exhibit perfect resistance in one direction and perfect conductance in the other, creating the possibility of using them for quantum data storage. The material contains only 3 parts per million of nitrogen. The diamond was grown on a stepped substrate, which eliminated cracking.
Surface property
Diamonds are naturally lipophilic
Lipophilicity (from Greek language, Greek λίπος "fat" and :wikt:φίλος, φίλος "friendly") is the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. Such compounds are c ...
and hydrophobic
In chemistry, hydrophobicity is the chemical property of a molecule (called a hydrophobe) that is seemingly repelled from a mass of water. In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, thu ...
, which means the diamonds' surface cannot be wet by water, but can be easily wet and stuck by oil. This property can be utilized to extract diamonds using oil when making synthetic diamonds. However, when diamond surfaces are chemically modified with certain ions, they are expected to become so hydrophilic
A hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water.Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon'' Oxford: Clarendon Press.
In contrast, hydrophobes are n ...
that they can stabilize multiple layers of water ice Water ice may refer to:
*Ice formed by water (as opposed to other substances)
*In ice climbing, ice made from flowing water (as opposed to ice from precipitation)
*The alternate term for various similar frozen fruit-flavoured desserts:
** Italian ic ...
at human body temperature
Normal human body temperature (normothermia, euthermia) is the typical temperature range found in humans. The normal human body temperature range is typically stated as .
Human body temperature varies. It depends on sex, age, time of day, exert ...
.
The surface of diamonds is partially oxidized. The oxidized surface can be reduced by heat treatment under hydrogen flow. That is to say, this heat treatment partially removes oxygen-containing functional groups. But diamonds (sp3C) are unstable against high temperature (above about ) under atmospheric pressure. The structure gradually changes into sp2C above this temperature. Thus, diamonds should be reduced below this temperature.
Chemical stability
At room temperature, diamonds do not react with any chemical reagents including strong acids and bases.
In an atmosphere of pure oxygen, diamond has an ignition point
The fire point, or combustion point, of a fuel
A fuel is any material that can be made to react with other substances so that it releases energy as thermal energy or to be used for work (physics), work. The concept was originally applied s ...
that ranges from to ; smaller crystals tend to burn more easily. It increases in temperature from red to white heat and burns with a pale blue flame, and continues to burn after the source of heat is removed. By contrast, in air the combustion will cease as soon as the heat is removed because the oxygen is diluted with nitrogen. A clear, flawless, transparent diamond is completely converted to carbon dioxide; any impurities will be left as ash. Heat generated from cutting a diamond will not ignite the diamond, and neither will a cigarette lighter, but house fires and blow torches are hot enough. Jewelers must be careful when molding the metal in a diamond ring.
Diamond powder of an appropriate grain size (around 50microns) burns with a shower of sparks after ignition from a flame. Consequently, pyrotechnic composition
A pyrotechnic composition is a substance or mixture of substances designed to produce an effect by heat, light, sound, gas/smoke or a combination of these, as a result of non-detonative self-sustaining exothermic reaction, exothermic chemical reac ...
s based on synthetic diamond
A synthetic diamond or laboratory-grown diamond (LGD), also called a lab-grown, laboratory-created, man-made, artisan-created, artificial, or cultured diamond, is a diamond that is produced in a controlled technological process, in contrast to ...
powder can be prepared. The resulting sparks are of the usual red-orange color, comparable to charcoal, but show a very linear trajectory which is explained by their high density. Diamond also reacts with fluorine gas above about .
Color
 Diamond has a wide
Diamond has a wide band gap
In solid-state physics and solid-state chemistry, a band gap, also called a bandgap or energy gap, is an energy range in a solid where no electronic states exist. In graphs of the electronic band structure of solids, the band gap refers to t ...
of corresponding to the deep ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of ...
wavelength of 225nanometers. This means that pure diamond should transmit visible light and appear as a clear colorless crystal. Colors in diamond originate from lattice defects and impurities. The diamond crystal lattice is exceptionally strong, and only atoms of nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
, boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three ...
, and hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
can be introduced into diamond during the growth at significant concentrations (up to atomic percents). Transition metals nickel
Nickel is a chemical element; it has symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive, but large pieces are slo ...
and cobalt
Cobalt is a chemical element; it has Symbol (chemistry), symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. ...
, which are commonly used for growth of synthetic diamond by high-pressure high-temperature techniques, have been detected in diamond as individual atoms; the maximum concentration is 0.01% for nickel and even less for cobalt. Virtually any element can be introduced to diamond by ion implantation.
Nitrogen is by far the most common impurity found in gem diamonds and is responsible for the yellow and brown color in diamonds. Boron is responsible for the blue color. Color in diamond has two additional sources: irradiation (usually by alpha particles), that causes the color in green diamonds, and plastic deformation
In engineering, deformation (the change in size or shape of an object) may be ''elastic'' or ''plastic''.
If the deformation is negligible, the object is said to be ''rigid''.
Main concepts
Occurrence of deformation in engineering application ...
of the diamond crystal lattice. Plastic deformation is the cause of color in some brown and perhaps pink and red diamonds. In order of increasing rarity, yellow diamond is followed by brown, colorless, then by blue, green, black, pink, orange, purple, and red.[ "Black", or ]carbonado
Carbonado, commonly known as black diamond, is one of the toughest forms of natural diamond. It is an impure, high-density, micro-porous form of polycrystalline diamond consisting of diamond, graphite, and amorphous carbon, with minor crystall ...
, diamonds are not truly black, but rather contain numerous dark inclusions that give the gems their dark appearance. Colored diamonds contain impurities or structural defects that cause the coloration, while pure or nearly pure diamonds are transparent and colorless. Most diamond impurities replace a carbon atom in the crystal lattice
In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystal, crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that ...
, known as a carbon flaw
A carbon flaw is a blemish present within a diamond crystalline form of carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms a ...
. The most common impurity, nitrogen, causes a slight to intense yellow coloration depending upon the type and concentration of nitrogen present.[ The ]Gemological Institute of America
The Gemological Institute of America (GIA) is a nonprofit institute based in Carlsbad, California. It is dedicated to research and education in the field of gemology and the jewelry arts. Founded in 1931, GIA's mission is to protect buyers and s ...
(GIA) classifies low saturation yellow and brown diamonds as diamonds in the ''normal color range'', and applies a grading scale from "D" (colorless) to "Z" (light yellow). Yellow diamonds of high color saturation or a different color, such as pink or blue, are called ''fancy colored'' diamonds and fall under a different grading scale.[
In 2008, the Wittelsbach Diamond, a ]blue diamond
Blue diamond is a type of diamond which exhibits all of the same inherent properties of the mineral except with the additional element of blue color in the stone. They are colored blue by trace impurities of boron within the crystalline lattice s ...
once belonging to the King of Spain, fetched over US$24 million at a Christie's auction. In May 2009, a blue diamond
Blue diamond is a type of diamond which exhibits all of the same inherent properties of the mineral except with the additional element of blue color in the stone. They are colored blue by trace impurities of boron within the crystalline lattice s ...
fetched the highest price per carat ever paid for a diamond when it was sold at auction for 10.5 million Swiss francs (6.97 million euros, or US$9.5 million at the time). That record was, however, beaten the same year: a vivid pink diamond was sold for US$10.8 million in Hong Kong on December 1, 2009.
Clarity
Clarity is one of the 4C's (color, clarity, cut and carat weight) that helps in identifying the quality of diamonds. The Gemological Institute of America
The Gemological Institute of America (GIA) is a nonprofit institute based in Carlsbad, California. It is dedicated to research and education in the field of gemology and the jewelry arts. Founded in 1931, GIA's mission is to protect buyers and s ...
(GIA) developed 11 clarity scales to decide the quality of a diamond for its sale value. The GIA clarity scale spans from Flawless (FL) to included (I) having internally flawless (IF), very, very slightly included (VVS), very slightly included (VS) and slightly included (SI) in between. Impurities in natural diamonds are due to the presence of natural minerals and oxides. The clarity scale grades the diamond based on the color, size, location of impurity and quantity of clarity visible under 10x magnification. Inclusions in diamond can be extracted by optical methods. The process is to take pre-enhancement images, identifying the inclusion removal part and finally removing the diamond facets and noises.
Fluorescence
 Between 25% and 35% of natural diamonds exhibit some degree of fluorescence when examined under invisible long-wave ultraviolet light or higher energy radiation sources such as X-rays and lasers. Incandescent lighting will not cause a diamond to fluoresce. Diamonds can fluoresce in a variety of colors including blue (most common), orange, yellow, white, green and very rarely red and purple. Although the causes are not well understood, variations in the atomic structure, such as the number of nitrogen atoms present are thought to contribute to the phenomenon.
Between 25% and 35% of natural diamonds exhibit some degree of fluorescence when examined under invisible long-wave ultraviolet light or higher energy radiation sources such as X-rays and lasers. Incandescent lighting will not cause a diamond to fluoresce. Diamonds can fluoresce in a variety of colors including blue (most common), orange, yellow, white, green and very rarely red and purple. Although the causes are not well understood, variations in the atomic structure, such as the number of nitrogen atoms present are thought to contribute to the phenomenon.
Thermal conductivity
Diamonds can be identified by their high thermal conductivity (900–). Their high refractive index
In optics, the refractive index (or refraction index) of an optical medium is the ratio of the apparent speed of light in the air or vacuum to the speed in the medium. The refractive index determines how much the path of light is bent, or refrac ...
is also indicative, but other materials have similar refractivity.
Geology
Diamonds are extremely rare, with concentrations of at most parts per billion in source rock.[ Before the 20th century, most diamonds were found in ]alluvial deposit
Alluvium (, ) is loose clay, silt, sand, or gravel that has been deposited by running water in a stream bed, on a floodplain, in an alluvial fan or beach, or in similar settings. Alluvium is also sometimes called alluvial deposit. Alluvium is ...
s. Loose diamonds are also found along existing and ancient shore
A coast (coastline, shoreline, seashore) is the land next to the sea or the line that forms the boundary between the land and the ocean or a lake. Coasts are influenced by the topography of the surrounding landscape and by aquatic erosion, su ...
lines, where they tend to accumulate because of their size and density.glacial till
image:Geschiebemergel.JPG, Closeup of glacial till. Note that the larger grains (pebbles and gravel) in the till are completely surrounded by the matrix of finer material (silt and sand), and this characteristic, known as ''matrix support'', is d ...
(notably in Wisconsin
Wisconsin ( ) is a U.S. state, state in the Great Lakes region, Great Lakes region of the Upper Midwest of the United States. It borders Minnesota to the west, Iowa to the southwest, Illinois to the south, Lake Michigan to the east, Michig ...
and Indiana
Indiana ( ) is a U.S. state, state in the Midwestern United States, Midwestern region of the United States. It borders Lake Michigan to the northwest, Michigan to the north and northeast, Ohio to the east, the Ohio River and Kentucky to the s ...
), but these deposits are not of commercial quality.[ These types of deposit were derived from localized igneous ]intrusions
In geology, an igneous intrusion (or intrusive body or simply intrusion) is a body of Intrusive rock, intrusive igneous rock that forms by crystallization of magma slowly cooling below the surface of the Earth. Intrusions have a wide variety o ...
through weathering
Weathering is the deterioration of rocks, soils and minerals (as well as wood and artificial materials) through contact with water, atmospheric gases, sunlight, and biological organisms. It occurs '' in situ'' (on-site, with little or no move ...
and transport
Transport (in British English) or transportation (in American English) is the intentional Motion, movement of humans, animals, and cargo, goods from one location to another. Mode of transport, Modes of transport include aviation, air, land tr ...
by wind
Wind is the natural movement of atmosphere of Earth, air or other gases relative to a planetary surface, planet's surface. Winds occur on a range of scales, from thunderstorm flows lasting tens of minutes, to local breezes generated by heatin ...
or water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
.Earth's mantle
Earth's mantle is a layer of silicate mineral, silicate rock between the Earth's crust, crust and the Earth's outer core, outer core. It has a mass of and makes up 67% of the mass of Earth. It has a thickness of making up about 46% of Earth's ...
, and most of this section discusses those diamonds. However, there are other sources. Some blocks of the crust, or terrane
In geology, a terrane (; in full, a tectonostratigraphic terrane) is a crust fragment formed on a tectonic plate (or broken off from it) and accreted or " sutured" to crust lying on another plate. The crustal block or fragment preserves its d ...
s, have been buried deep enough as the crust thickened so they experienced ultra-high-pressure metamorphism Ultra-high-pressure metamorphism refers to metamorphic processes at pressures high enough to stabilize coesite, the high-pressure polymorph of SiO2. It is important because the processes that form and exhume ultra-high-pressure (UHP) metamorphic r ...
. These have evenly distributed ''microdiamonds'' that show no sign of transport by magma. In addition, when meteorites strike the ground, the shock wave can produce high enough temperatures and pressures for ''microdiamonds'' and ''nanodiamonds
Nanodiamonds, or diamond nanoparticles, are diamonds with a size below 100 nanometers. They can be produced by impact events such as an explosion or meteoritic impacts. Because of their inexpensive, large-scale synthesis, potential for surfac ...
'' to form.[ Impact-type microdiamonds can be used as an indicator of ancient impact craters. ]Popigai impact structure
The Popigai impact structure is the eroded remnant of an impact crater in northern Siberia, Russia. It is tied with the Acraman impact structure as the fourth largest verified impact structure on Earth. A large bolide impact created the c ...
in Russia may have the world's largest diamond deposit, estimated at trillions of carats, and formed by an asteroid impact.
A common misconception is that diamonds form from highly compressed coal
Coal is a combustible black or brownish-black sedimentary rock, formed as rock strata called coal seams. Coal is mostly carbon with variable amounts of other Chemical element, elements, chiefly hydrogen, sulfur, oxygen, and nitrogen.
Coal i ...
. Coal is formed from buried prehistoric plants, and most diamonds that have been dated are far older than the first embryophyte, land plants. It is possible that diamonds can form from coal in subduction zones, but diamonds formed in this way are rare, and the carbon source is more likely carbonate rocks and organic carbon in sediments, rather than coal.
Surface distribution
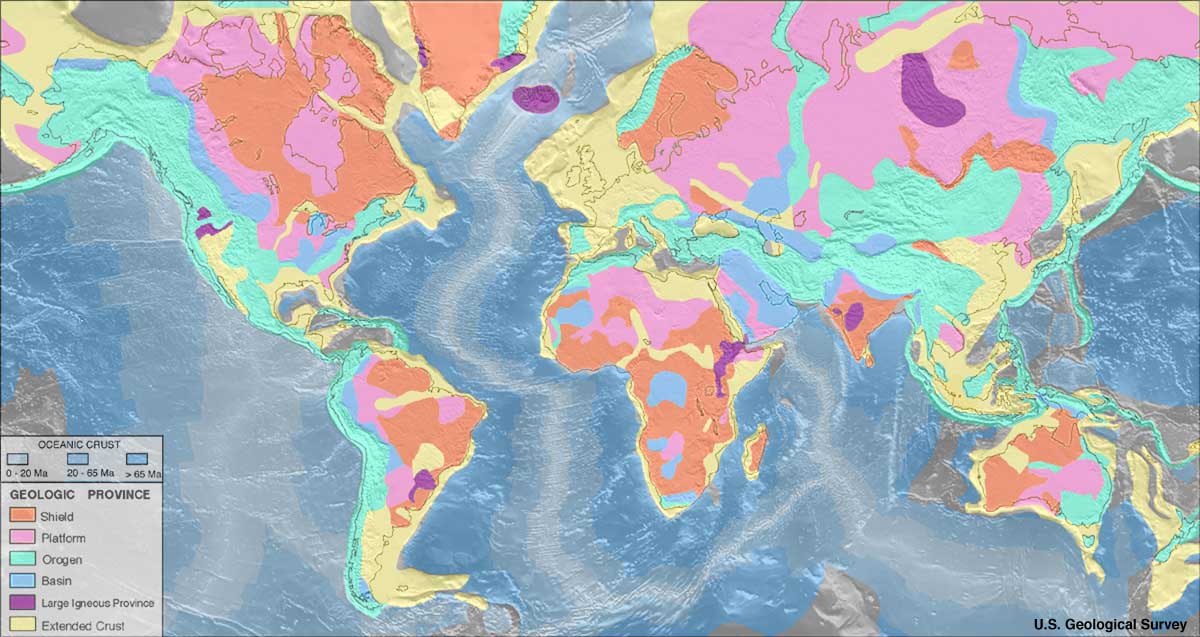 Diamonds are far from evenly distributed over the Earth. A rule of thumb known as Clifford's rule states that they are almost always found in
Diamonds are far from evenly distributed over the Earth. A rule of thumb known as Clifford's rule states that they are almost always found in kimberlite
Kimberlite is an igneous rock and a rare variant of peridotite. It is most commonly known as the main host matrix for diamonds. It is named after the town of Kimberley, Northern Cape, Kimberley in South Africa, where the discovery of an 83.5-Car ...
s on the oldest part of cratons, the stable cores of continents with typical ages of 2.5billion years or more.[ However, there are exceptions. The Argyle diamond mine in Australia, the largest producer of diamonds by weight in the world, is located in a ''mobile belt'', also known as an ''orogeny, orogenic belt'', a weaker zone surrounding the central craton that has undergone compressional tectonics. Instead of ]kimberlite
Kimberlite is an igneous rock and a rare variant of peridotite. It is most commonly known as the main host matrix for diamonds. It is named after the town of Kimberley, Northern Cape, Kimberley in South Africa, where the discovery of an 83.5-Car ...
, the host rock is lamproite
Lamproite is an ultrapotassic mantle-derived volcanic or subvolcanic rock. It has low CaO, Al2O3, Na2O, high K2O/Al2O3, a relatively high MgO content and extreme enrichment in incompatible elements.
Lamproites are geographically widespre ...
. Lamproites with diamonds that are not economically viable are also found in the United States, India, and Australia.[ In addition, diamonds in the Algoman orogeny, Wawa belt of the Superior craton, Superior province in Canada and microdiamonds in the Northeastern Japan arc, island arc of Japan are found in a type of rock called lamprophyre.][
Kimberlites can be found in narrow (1 to 4 meters) dikes and sills, and in pipes with diameters that range from about 75 m to 1.5 km. Fresh rock is dark bluish green to greenish gray, but after exposure rapidly turns brown and crumbles. It is hybrid rock with a chaotic mixture of small minerals and rock fragments (clastic rock, clasts) up to the size of watermelons. They are a mixture of xenocrysts and xenoliths (minerals and rocks carried up from the lower crust and mantle), pieces of surface rock, altered minerals such as Serpentine subgroup, serpentine, and new minerals that crystallized during the eruption. The texture varies with depth. The composition forms a continuum with carbonatites, but the latter have too much oxygen for carbon to exist in a pure form. Instead, it is locked up in the mineral calcite ().][
All three of the diamond-bearing rocks (kimberlite, lamproite and lamprophyre) lack certain minerals (melilite and kalsilite) that are incompatible with diamond formation. In ]kimberlite
Kimberlite is an igneous rock and a rare variant of peridotite. It is most commonly known as the main host matrix for diamonds. It is named after the town of Kimberley, Northern Cape, Kimberley in South Africa, where the discovery of an 83.5-Car ...
, olivine is large and conspicuous, while lamproite has Ti-phlogopite and lamprophyre has biotite and amphibole. They are all derived from magma types that erupt rapidly from small amounts of melt, are rich in Volatility (chemistry), volatiles and magnesium oxide, and are less redox, oxidizing than more common mantle melts such as basalt. These characteristics allow the melts to carry diamonds to the surface before they dissolve.[
]
Exploration
Kimberlite pipes can be difficult to find. They weather quickly (within a few years after exposure) and tend to have lower topographic relief than surrounding rock. If they are visible in outcrops, the diamonds are never visible because they are so rare. In any case, kimberlites are often covered with vegetation, sediments, soils, or lakes. In modern searches, geophysical survey, geophysical methods such as aeromagnetic surveys, electrical resistivity tomography, electrical resistivity, and gravimetry, help identify promising regions to explore. This is aided by isotopic dating and modeling of the geological history. Then surveyors must go to the area and collect samples, looking for kimberlite fragments or ''indicator minerals''. The latter have compositions that reflect the conditions where diamonds form, such as extreme melt depletion or high pressures in eclogites. However, indicator minerals can be misleading; a better approach is geothermobarometry, where the compositions of minerals are analyzed as if they were in equilibrium with mantle minerals.[
Finding kimberlites requires persistence, and only a small fraction contain diamonds that are commercially viable. The only major discoveries since about 1980 have been in Canada. Since existing mines have lifetimes of as little as 25 years, there could be a shortage of new natural diamonds in the future.][
]
Ages
Diamonds are dated by analyzing inclusions using the decay of radioactive isotopes. Depending on the elemental abundances, one can look at the decay of Rubidium–strontium dating, rubidium to strontium, Samarium–neodymium dating, samarium to neodymium, Uranium–lead dating, uranium to lead, Argon–argon dating, argon-40 to argon-39, or Rhenium–osmium dating, rhenium to osmium. Those found in kimberlites have ages ranging from , and there can be multiple ages in the same kimberlite, indicating multiple episodes of diamond formation. The kimberlites themselves are much younger. Most of them have ages between tens of millions and 300 million years old, although there are some older exceptions (Argyle, Premier Mine, Premier and Wawa). Thus, the kimberlites formed independently of the diamonds and served only to transport them to the surface.[ Kimberlites are also much younger than the cratons they have erupted through. The reason for the lack of older kimberlites is unknown, but it suggests there was some change in mantle chemistry or tectonics. No kimberlite has erupted in human history.][
]
Origin in mantle
Most gem-quality diamonds come from depths of 150–250 km in the lithosphere. Such depths occur below cratons in ''mantle keels'', the thickest part of the lithosphere. These regions have high enough pressure and temperature to allow diamonds to form and they are not convecting, so diamonds can be stored for billions of years until a kimberlite eruption samples them.[
Host rocks in a mantle keel include harzburgite and lherzolite, two type of peridotite. The most dominant rock type in the upper mantle (Earth), upper mantle, peridotite is an ]igneous rock
Igneous rock ( ), or magmatic rock, is one of the three main rock types, the others being sedimentary and metamorphic. Igneous rocks are formed through the cooling and solidification of magma or lava.
The magma can be derived from partial ...
consisting mostly of the minerals olivine and pyroxene; it is low in Silicon dioxide, silica and high in magnesium. However, diamonds in peridotite rarely survive the trip to the surface.[ Another common source that does keep diamonds intact is eclogite, a metamorphic rock that typically forms from basalt as an oceanic plate plunges into the mantle at a Subduction, subduction zone.][
A smaller fraction of diamonds (about 150 have been studied) come from depths of 330–660 km, a region that includes the Transition zone (Earth), transition zone. They formed in eclogite but are distinguished from diamonds of shallower origin by inclusions of majorite (a form of garnet with excess silicon). A similar proportion of diamonds comes from the lower mantle at depths between 660 and 800 km.][
Diamond is thermodynamically stable at high pressures and temperatures, with the phase transition from ]graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable ...
occurring at greater temperatures as the pressure increases. Thus, underneath continents it becomes stable at temperatures of 950degrees Celsius and pressures of 4.5 gigapascals, corresponding to depths of 150kilometers or greater. In subduction zones, which are colder, it becomes stable at temperatures of 800 °C and pressures of 3.5gigapascals. At depths greater than 240 km, iron–nickel metal phases are present and carbon is likely to be either dissolved in them or in the form of carbides. Thus, the deeper origin of some diamonds may reflect unusual growth environments.[
In 2018 the first known natural samples of a phase of ice called Ice VII were found as inclusions in diamond samples. The inclusions formed at depths between 400 and 800 km, straddling the upper and lower mantle, and provide evidence for water-rich fluid at these depths.]
Carbon sources
The mantle has roughly one billion tonne, gigatonnes of carbon (for comparison, the atmosphere-ocean system has about 44,000 gigatonnes). Carbon has two stable nuclide, stable isotopes, carbon-12, 12C and carbon-13, 13C, in a ratio of approximately 99:1 by mass.[ This ratio has a wide range in meteorites, which implies that it also varied a lot in the early Earth. It can also be altered by surface processes like photosynthesis. The fraction is generally compared to a standard sample using a ratio isotopic signature#Carbon isotopes, δ13C expressed in parts per thousand. Common rocks from the mantle such as basalts, carbonatites, and kimberlites have ratios between −8 and −2. On the surface, organic sediments have an average of −25 while carbonates have an average of 0.][
Populations of diamonds from different sources have distributions of δ13C that vary markedly. Peridotitic diamonds are mostly within the typical mantle range; eclogitic diamonds have values from −40 to +3, although the peak of the distribution is in the mantle range. This variability implies that they are not formed from carbon that is ''primordial'' (having resided in the mantle since the Earth formed). Instead, they are the result of tectonic processes, although (given the ages of diamonds) not necessarily the same tectonic processes that act in the present.][ Diamond-forming carbon originates in the top 700 kilometers (430 mi) or so of the upper mantle closest to the surface, known as the asthenosphere.][
]
Formation and growth
 Diamonds in the mantle form through a ''metasomatism, metasomatic'' process where a C–O–H–N–S fluid or melt dissolves minerals in a rock and replaces them with new minerals. (The vague term C–O–H–N–S is commonly used because the exact composition is not known.) Diamonds form from this fluid either by reduction of oxidized carbon (e.g., CO2 or CO3) or oxidation of a reduced phase such as methane.
Diamonds in the mantle form through a ''metasomatism, metasomatic'' process where a C–O–H–N–S fluid or melt dissolves minerals in a rock and replaces them with new minerals. (The vague term C–O–H–N–S is commonly used because the exact composition is not known.) Diamonds form from this fluid either by reduction of oxidized carbon (e.g., CO2 or CO3) or oxidation of a reduced phase such as methane.[
Using probes such as polarized light, photoluminescence, and cathodoluminescence, a series of growth zones can be identified in diamonds. The characteristic pattern in diamonds from the lithosphere involves a nearly concentric series of zones with very thin oscillations in luminescence and alternating episodes where the carbon is resorbed by the fluid and then grown again. Diamonds from below the lithosphere have a more irregular, almost polycrystalline texture, reflecting the higher temperatures and pressures as well as the transport of the diamonds by convection.][
]
Transport to the surface
 Geological evidence supports a model in which kimberlite magma rises at 4–20 meters per second, creating an upward path by hydraulic fracturing of the rock. As the pressure decreases, a vapor phase exsolution, exsolves from the magma, and this helps to keep the magma fluid. At the surface, the initial eruption explodes out through fissures at high speeds (over ). Then, at lower pressures, the rock is eroded, forming a pipe and producing fragmented rock (breccia). As the eruption wanes, there is Pyroclastic rock, pyroclastic phase and then metamorphism and hydration produces serpentinites.
Geological evidence supports a model in which kimberlite magma rises at 4–20 meters per second, creating an upward path by hydraulic fracturing of the rock. As the pressure decreases, a vapor phase exsolution, exsolves from the magma, and this helps to keep the magma fluid. At the surface, the initial eruption explodes out through fissures at high speeds (over ). Then, at lower pressures, the rock is eroded, forming a pipe and producing fragmented rock (breccia). As the eruption wanes, there is Pyroclastic rock, pyroclastic phase and then metamorphism and hydration produces serpentinites.[
]
Double diamonds
In rare cases, diamonds have been found that contain a cavity within which is a second diamond. The first double diamond, the Matryoshka (diamond), Matryoshka, was found by Alrosa in Sakha Republic, Yakutia, Russia, in 2019. Another one was found in the Ellendale Diamond Field in Western Australia in 2021.
In space
Although diamonds on Earth are rare, they are very common in space. In meteorites, about three percent of the carbon is in the form of nanodiamonds, having diameters of a few nanometers. Sufficiently small diamonds can form in the cold of space because their lower surface energy makes them more stable than graphite. The isotopic signatures of some nanodiamonds indicate they were formed outside the Solar System in stars.
High pressure experiments predict that large quantities of diamonds condense from methane into a "diamond rain" on the ice giant planets Uranus
Uranus is the seventh planet from the Sun. It is a gaseous cyan-coloured ice giant. Most of the planet is made of water, ammonia, and methane in a Supercritical fluid, supercritical phase of matter, which astronomy calls "ice" or Volatile ( ...
and Neptune
Neptune is the eighth and farthest known planet from the Sun. It is the List of Solar System objects by size, fourth-largest planet in the Solar System by diameter, the third-most-massive planet, and the densest giant planet. It is 17 t ...
. Some extrasolar planets may be almost entirely composed of diamond.
Diamonds may exist in carbon-rich stars, particularly white dwarfs. One theory for the origin of carbonado
Carbonado, commonly known as black diamond, is one of the toughest forms of natural diamond. It is an impure, high-density, micro-porous form of polycrystalline diamond consisting of diamond, graphite, and amorphous carbon, with minor crystall ...
, the toughest form of diamond, is that it originated in a white dwarf or supernova. Diamonds formed in stars may have been the first minerals.
Industry
 The most familiar uses of diamonds today are as gemstones used for adornment, and as industrial abrasives for cutting hard materials. The markets for gem-grade and industrial-grade diamonds value diamonds differently.
The most familiar uses of diamonds today are as gemstones used for adornment, and as industrial abrasives for cutting hard materials. The markets for gem-grade and industrial-grade diamonds value diamonds differently.
Gem-grade diamonds
The Dispersion (optics), dispersion of white light into spectral colors is the primary gemological characteristic of gem diamonds. In the 20th century, experts in gemology developed methods of grading diamonds and other gemstones based on the characteristics most important to their value as a gem. Four characteristics, known informally as the ''four Cs'', are now commonly used as the basic descriptors of diamonds: these are its mass in ''Carat (unit), carats'' (a carat being equal to 0.2grams), ''Diamond cut, cut'' (quality of the cut is graded according to aspect ratio, proportions, symmetry and polishing, polish), ''Diamond color, color'' (how close to white or colorless; for fancy diamonds how intense is its hue), and ''Diamond clarity, clarity'' (how free is it from inclusion (mineral), inclusions). A large, flawless diamond is known as a Paragon (diamond), paragon.
A large trade in gem-grade diamonds exists. Although most gem-grade diamonds are sold newly polished, there is a well-established market for resale of polished diamonds (e.g. pawnbroking, auctions, second-hand jewelry stores, diamantaires, bourses, etc.). One hallmark of the trade in gem-quality diamonds is its remarkable concentration: wholesale trade and diamond cutting is limited to just a few locations; in 2003, 92% of the world's diamonds were cut and polished in Surat, India
India, officially the Republic of India, is a country in South Asia. It is the List of countries and dependencies by area, seventh-largest country by area; the List of countries by population (United Nations), most populous country since ...
. Other important centers of diamond cutting and trading are the Antwerp diamond district in Belgium, where the International Gemological Institute is based, London, the Diamond District in New York City, the Diamond Exchange District in Tel Aviv and Amsterdam. One contributory factor is the geological nature of diamond deposits: several large primary kimberlite-pipe mines each account for significant portions of market share (such as the Jwaneng diamond mine, Jwaneng mine in Botswana, which is a single large-pit mine that can produce between of diamonds per year). Secondary alluvial diamond deposits, on the other hand, tend to be fragmented amongst many different operators because they can be dispersed over many hundreds of square kilometers (e.g., alluvial deposits in Brazil).
The production and distribution of diamonds is largely consolidated in the hands of a few key players, and concentrated in traditional diamond trading centers, the most important being Antwerp, where 80% of all rough diamonds, 50% of all cut diamonds and more than 50% of all rough, cut and industrial diamonds combined are handled. Further down the supply chain, members of The World Federation of Diamond Bourses (WFDB) act as a medium for wholesale diamond exchange, trading both polished and rough diamonds. The WFDB consists of independent diamond bourses in major cutting centers such as Tel Aviv, Antwerp, Johannesburg and other cities across the US, Europe and Asia.
Further down the supply chain, members of The World Federation of Diamond Bourses (WFDB) act as a medium for wholesale diamond exchange, trading both polished and rough diamonds. The WFDB consists of independent diamond bourses in major cutting centers such as Tel Aviv, Antwerp, Johannesburg and other cities across the US, Europe and Asia.[ In 2000, the WFDB and The International Diamond Manufacturers Association established the World Diamond Council to prevent the trading of diamonds used to fund war and inhumane acts. WFDB's additional activities include sponsoring the World Diamond Congress every two years, as well as the establishment of the International Diamond Council (IDC) to oversee diamond grading.
Once purchased by Sightholders (which is a trademark term referring to the companies that have a three-year supply contract with DTC), diamonds are cut and polished in preparation for sale as gemstones ('industrial' stones are regarded as a by-product of the gemstone market; they are used for abrasives).][ Traditional diamond cutting centers are Antwerp, Amsterdam, Johannesburg, New York City, and Tel Aviv. Recently, diamond cutting centers have been established in China, India, Thailand, Namibia and Botswana.][ Cutting centers with lower cost of labor, notably Surat in Gujarat, Gujarat, India, handle a larger number of smaller carat diamonds, while smaller quantities of larger or more valuable diamonds are more likely to be handled in Europe or North America. The recent expansion of this industry in India, employing low cost labor, has allowed smaller diamonds to be prepared as gems in greater quantities than was previously economically feasible.]
Cutting
Mined rough diamonds are converted into gems through a multi-step process called "cutting". Diamonds are extremely hard, but also brittle and can be split up by a single blow. Therefore, diamond cutting is traditionally considered as a delicate procedure requiring skills, scientific knowledge, tools and experience. Its final goal is to produce a faceted jewel where the specific angles between the facets would optimize the diamond luster, that is dispersion of white light, whereas the number and area of facets would determine the weight of the final product. The weight reduction upon cutting is significant and can be of the order of 50%.[
After initial cutting, the diamond is shaped in numerous stages of polishing. Unlike cutting, which is a responsible but quick operation, polishing removes material by gradual erosion and is extremely time-consuming. The associated technique is well developed; it is considered as a routine and can be performed by technicians. After polishing, the diamond is reexamined for possible flaws, either remaining or induced by the process. Those flaws are concealed through various diamond enhancement techniques, such as repolishing, crack filling, or clever arrangement of the stone in the jewelry. Remaining non-diamond inclusions are removed through laser drilling and filling of the voids produced.]
Marketing
 Marketing has significantly affected the image of diamond as a valuable commodity.
N. W. Ayer & Son, the advertising firm retained by De Beers in the mid-20th century, succeeded in reviving the American diamond market and the firm created new markets in countries where no diamond tradition had existed before. N. W. Ayer's marketing included product placement, advertising focused on the diamond product itself rather than the De Beers brand, and associations with celebrities and royalty. Without advertising the De Beers brand, De Beers was advertising its competitors' diamond products as well, but this was not a concern as De Beers dominated the diamond market throughout the 20th century. De Beers' market share dipped temporarily to second place in the global market below Alrosa in the aftermath of the global economic crisis of 2008, down to less than 29% in terms of carats mined, rather than sold.
Marketing has significantly affected the image of diamond as a valuable commodity.
N. W. Ayer & Son, the advertising firm retained by De Beers in the mid-20th century, succeeded in reviving the American diamond market and the firm created new markets in countries where no diamond tradition had existed before. N. W. Ayer's marketing included product placement, advertising focused on the diamond product itself rather than the De Beers brand, and associations with celebrities and royalty. Without advertising the De Beers brand, De Beers was advertising its competitors' diamond products as well, but this was not a concern as De Beers dominated the diamond market throughout the 20th century. De Beers' market share dipped temporarily to second place in the global market below Alrosa in the aftermath of the global economic crisis of 2008, down to less than 29% in terms of carats mined, rather than sold.
Industrial-grade diamonds


 Industrial diamonds are valued mostly for their hardness and thermal conductivity, making many of the gemological characteristics of diamonds, such as the 4 Cs, irrelevant for most applications. Eighty percent of mined diamonds (equal to about annually) are unsuitable for use as gemstones and are used industrially. In addition to mined diamonds, synthetic diamonds found industrial applications almost immediately after their invention in the 1950s; in 2014, of synthetic diamonds were produced, 90% of which were produced in China. Approximately 90% of diamond Grinding (abrasive cutting), grinding grit is currently of synthetic origin.
Industrial diamonds are valued mostly for their hardness and thermal conductivity, making many of the gemological characteristics of diamonds, such as the 4 Cs, irrelevant for most applications. Eighty percent of mined diamonds (equal to about annually) are unsuitable for use as gemstones and are used industrially. In addition to mined diamonds, synthetic diamonds found industrial applications almost immediately after their invention in the 1950s; in 2014, of synthetic diamonds were produced, 90% of which were produced in China. Approximately 90% of diamond Grinding (abrasive cutting), grinding grit is currently of synthetic origin.[ With the continuing advances being made in the production of synthetic diamonds, future applications are becoming feasible. The high ]thermal conductivity
The thermal conductivity of a material is a measure of its ability to heat conduction, conduct heat. It is commonly denoted by k, \lambda, or \kappa and is measured in W·m−1·K−1.
Heat transfer occurs at a lower rate in materials of low ...
of diamond makes it suitable as a heat sink for integrated circuits in electronics.
Mining
Approximately of diamonds are mined annually, with a total value of nearly US$9 billion, and about are synthesized annually.India
India, officially the Republic of India, is a country in South Asia. It is the List of countries and dependencies by area, seventh-largest country by area; the List of countries by population (United Nations), most populous country since ...
, Russia, Brazil, and Australia.[ They are mined from kimberlite and lamproite volcanic pipes, which can bring diamond crystals, originating from deep within the Earth where high pressures and temperatures enable them to form, to the surface. The mining and distribution of natural diamonds are subjects of frequent controversy such as concerns over the sale of ''blood diamonds'' or ''conflict diamonds'' by African paramilitary groups.][ the separation was done with grease belts; diamonds have a stronger tendency to stick to grease than the other minerals in the ore.]
 Historically, diamonds were found only in
Historically, diamonds were found only in alluvial deposit
Alluvium (, ) is loose clay, silt, sand, or gravel that has been deposited by running water in a stream bed, on a floodplain, in an alluvial fan or beach, or in similar settings. Alluvium is also sometimes called alluvial deposit. Alluvium is ...
s in Guntur district, Guntur and Krishna district of the Krishna River delta in Southern India. India led the world in diamond production from the time of their discovery in approximately the 9th century BC[ to the mid-18th century AD, but the commercial potential of these sources had been exhausted by the late 18th century and at that time India was eclipsed by Brazil where the first non-Indian diamonds were found in 1725.][ Currently, one of the most prominent Indian mines is located at Panna District, Panna.
Diamond extraction from primary deposits (kimberlites and lamproites) started in the 1870s after the discovery of the Diamond Fields in South Africa. Production has increased over time and now an accumulated total of have been mined since that date.][
In the U.S., diamonds have been found in Arkansas, Colorado, New Mexico, Wyoming, and Montana.][
Today, most commercially viable diamond deposits are in Russia (mostly in Sakha Republic, for example Mir Mine, Mir pipe and Udachnaya pipe), Botswana, Australia (Northern Australia, Northern and Western Australia) and the Democratic Republic of the Congo. In 2005, Russia produced almost one-fifth of the global diamond output, according to the British Geological Survey. Australia boasts the richest diamantiferous pipe, with production from the Argyle diamond mine reaching peak levels of 42metric tons per year in the 1990s.][ There are also commercial deposits being actively mined in the Northwest Territories of Canada and Brazil.][ Diamond prospectors continue to search the globe for diamond-bearing kimberlite and lamproite pipes.
]
Political issues
In some of the more politically unstable central African and west African countries, revolutionary groups have taken control of List of diamond mines, diamond mines, using proceeds from diamond sales to finance their operations. Diamonds sold through this process are known as ''conflict diamonds'' or ''blood diamonds''.[
In response to public concerns that their diamond purchases were contributing to war and human rights abuses, the United Nations, the diamond industry and diamond-trading nations introduced the Kimberley Process in 2002.][
The Canadian Government has set up a body known as the Canadian Diamond Code of Conduct to help authenticate Canadian diamonds. This is a stringent tracking system of diamonds and helps protect the "conflict free" label of Canadian diamonds.
Mineral resource exploitation in general causes irreversible environmental damage, which must be weighed against the socio-economic benefits to a country.
]
Synthetics, simulants, and enhancements
Synthetics
Synthetic diamonds are diamonds manufactured in a laboratory, as opposed to diamonds mined from the Earth. The gemological and industrial uses of diamond have created a large demand for rough stones. This demand has been satisfied in large part by synthetic diamonds, which have been manufactured by various processes for more than half a century. However, in recent years it has become possible to produce gem-quality synthetic diamonds of significant size.nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
impurities. Other colors may also be reproduced such as blue, green or pink, which are a result of the addition of boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three ...
or from Gemstone irradiation, irradiation after synthesis.
Another popular method of growing synthetic diamond is chemical vapor deposition
Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high-quality, and high-performance, solid materials. The process is often used in the semiconductor industry to produce thin films.
In typical CVD, the wafer (electro ...
(CVD). The growth occurs under low pressure (below atmospheric pressure). It involves feeding a mixture of gases (typically to hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
) into a chamber and splitting them into chemically active radical (chemistry), radicals in a plasma (physics), plasma ignited by microwaves, hot filament, electric arc, arc discharge, welding torch, or laser. This method is mostly used for coatings, but can also produce single crystals several millimeters in size (see picture).[
As of 2010, nearly all 5,000 million carats (1,000tonnes) of synthetic diamonds produced per year are for industrial use. Around 50% of the 133 million carats of natural diamonds mined per year end up in industrial use.]
Simulants
 A diamond simulant is a non-diamond material that is used to simulate the appearance of a diamond, and may be referred to as diamante. Cubic zirconia is the most common. The gemstone moissanite (silicon carbide) can be treated as a diamond simulant, though more costly to produce than cubic zirconia. Both are produced synthetically.
A diamond simulant is a non-diamond material that is used to simulate the appearance of a diamond, and may be referred to as diamante. Cubic zirconia is the most common. The gemstone moissanite (silicon carbide) can be treated as a diamond simulant, though more costly to produce than cubic zirconia. Both are produced synthetically.
Enhancements
Diamond enhancements are specific treatments performed on natural or synthetic diamonds (usually those already cut and polished into a gem), which are designed to better the gemological characteristics of the stone in one or more ways. These include laser drilling to remove inclusions, application of sealants to fill cracks, treatments to improve a white diamond's color grade, and treatments to give fancy color to a white diamond.
Coatings are increasingly used to give a diamond simulant such as cubic zirconia a more "diamond-like" appearance. One such substance is diamond-like carbon—an amorphous carbonaceous material that has some physical properties similar to those of the diamond. Advertising suggests that such a coating would transfer some of these diamond-like properties to the coated stone, hence enhancing the diamond simulant. Techniques such as Raman spectroscopy should easily identify such a treatment.
Identification
Early diamond identification tests included a scratch test relying on the superior hardness of diamond. This test is destructive, as a diamond can scratch another diamond, and is rarely used nowadays. Instead, diamond identification relies on its superior thermal conductivity. Electronic thermal probes are widely used in the gemological centers to separate diamonds from their imitations. These probes consist of a pair of battery-powered thermistors mounted in a fine copper tip. One thermistor functions as a heating device while the other measures the temperature of the copper tip: if the stone being tested is a diamond, it will conduct the tip's thermal energy rapidly enough to produce a measurable temperature drop. This test takes about two to three seconds.
Whereas the thermal probe can separate diamonds from most of their simulants, distinguishing between various types of diamond, for example synthetic or natural, irradiated or non-irradiated, etc., requires more advanced, optical techniques. Those techniques are also used for some diamonds simulants, such as silicon carbide, which pass the thermal conductivity test. Optical techniques can distinguish between natural diamonds and synthetic diamonds. They can also identify the vast majority of treated natural diamonds.[
Laboratories use techniques such as spectroscopy, microscopy, and luminescence under shortwave ultraviolet light to determine a diamond's origin.][ They also use specially made instruments to aid them in the identification process. Two screening instruments are the ''DiamondSure'' and the ''DiamondView'', both produced by the Diamond Trading Company, DTC and marketed by the GIA.
Several methods for identifying synthetic diamonds can be performed, depending on the method of production and the color of the diamond. CVD diamonds can usually be identified by an orange fluorescence. D–J colored diamonds can be screened through the Swiss Gemmological Institute's]
See also
* Deep carbon cycle
* Diamondoid
* List of diamonds
** List of largest rough diamonds
* List of minerals
* Superhard material
Notes
Citations
General and cited references
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
Further reading
*
*
External links
Properties of diamond: Ioffe database
*
{{Authority control
Diamond,
Abrasives
Articles containing video clips
Crystals
Cubic minerals
Economic geology
Group IV semiconductors
Impact event minerals
Industrial minerals
Luminescent minerals
Minerals in space group 227
Native element minerals
Transparent materials
 Diamond is a solid form of the element carbon with its atoms arranged in a
Diamond is a solid form of the element carbon with its atoms arranged in a  The most common crystal structure of diamond is called
The most common crystal structure of diamond is called  Diamonds occur most often as
Diamonds occur most often as  Diamond is the hardest material on the qualitative
Diamond is the hardest material on the qualitative  Between 25% and 35% of natural diamonds exhibit some degree of fluorescence when examined under invisible long-wave ultraviolet light or higher energy radiation sources such as X-rays and lasers. Incandescent lighting will not cause a diamond to fluoresce. Diamonds can fluoresce in a variety of colors including blue (most common), orange, yellow, white, green and very rarely red and purple. Although the causes are not well understood, variations in the atomic structure, such as the number of nitrogen atoms present are thought to contribute to the phenomenon.
Between 25% and 35% of natural diamonds exhibit some degree of fluorescence when examined under invisible long-wave ultraviolet light or higher energy radiation sources such as X-rays and lasers. Incandescent lighting will not cause a diamond to fluoresce. Diamonds can fluoresce in a variety of colors including blue (most common), orange, yellow, white, green and very rarely red and purple. Although the causes are not well understood, variations in the atomic structure, such as the number of nitrogen atoms present are thought to contribute to the phenomenon.
 Diamonds are far from evenly distributed over the Earth. A rule of thumb known as Clifford's rule states that they are almost always found in
Diamonds are far from evenly distributed over the Earth. A rule of thumb known as Clifford's rule states that they are almost always found in  Diamonds in the mantle form through a ''metasomatism, metasomatic'' process where a C–O–H–N–S fluid or melt dissolves minerals in a rock and replaces them with new minerals. (The vague term C–O–H–N–S is commonly used because the exact composition is not known.) Diamonds form from this fluid either by reduction of oxidized carbon (e.g., CO2 or CO3) or oxidation of a reduced phase such as methane.
Using probes such as polarized light, photoluminescence, and cathodoluminescence, a series of growth zones can be identified in diamonds. The characteristic pattern in diamonds from the lithosphere involves a nearly concentric series of zones with very thin oscillations in luminescence and alternating episodes where the carbon is resorbed by the fluid and then grown again. Diamonds from below the lithosphere have a more irregular, almost polycrystalline texture, reflecting the higher temperatures and pressures as well as the transport of the diamonds by convection.
Diamonds in the mantle form through a ''metasomatism, metasomatic'' process where a C–O–H–N–S fluid or melt dissolves minerals in a rock and replaces them with new minerals. (The vague term C–O–H–N–S is commonly used because the exact composition is not known.) Diamonds form from this fluid either by reduction of oxidized carbon (e.g., CO2 or CO3) or oxidation of a reduced phase such as methane.
Using probes such as polarized light, photoluminescence, and cathodoluminescence, a series of growth zones can be identified in diamonds. The characteristic pattern in diamonds from the lithosphere involves a nearly concentric series of zones with very thin oscillations in luminescence and alternating episodes where the carbon is resorbed by the fluid and then grown again. Diamonds from below the lithosphere have a more irregular, almost polycrystalline texture, reflecting the higher temperatures and pressures as well as the transport of the diamonds by convection.
 Geological evidence supports a model in which kimberlite magma rises at 4–20 meters per second, creating an upward path by hydraulic fracturing of the rock. As the pressure decreases, a vapor phase exsolution, exsolves from the magma, and this helps to keep the magma fluid. At the surface, the initial eruption explodes out through fissures at high speeds (over ). Then, at lower pressures, the rock is eroded, forming a pipe and producing fragmented rock (breccia). As the eruption wanes, there is Pyroclastic rock, pyroclastic phase and then metamorphism and hydration produces serpentinites.
Geological evidence supports a model in which kimberlite magma rises at 4–20 meters per second, creating an upward path by hydraulic fracturing of the rock. As the pressure decreases, a vapor phase exsolution, exsolves from the magma, and this helps to keep the magma fluid. At the surface, the initial eruption explodes out through fissures at high speeds (over ). Then, at lower pressures, the rock is eroded, forming a pipe and producing fragmented rock (breccia). As the eruption wanes, there is Pyroclastic rock, pyroclastic phase and then metamorphism and hydration produces serpentinites.
 The most familiar uses of diamonds today are as gemstones used for adornment, and as industrial abrasives for cutting hard materials. The markets for gem-grade and industrial-grade diamonds value diamonds differently.
The most familiar uses of diamonds today are as gemstones used for adornment, and as industrial abrasives for cutting hard materials. The markets for gem-grade and industrial-grade diamonds value diamonds differently.
 Further down the supply chain, members of The World Federation of Diamond Bourses (WFDB) act as a medium for wholesale diamond exchange, trading both polished and rough diamonds. The WFDB consists of independent diamond bourses in major cutting centers such as Tel Aviv, Antwerp, Johannesburg and other cities across the US, Europe and Asia. In 2000, the WFDB and The International Diamond Manufacturers Association established the World Diamond Council to prevent the trading of diamonds used to fund war and inhumane acts. WFDB's additional activities include sponsoring the World Diamond Congress every two years, as well as the establishment of the International Diamond Council (IDC) to oversee diamond grading.
Once purchased by Sightholders (which is a trademark term referring to the companies that have a three-year supply contract with DTC), diamonds are cut and polished in preparation for sale as gemstones ('industrial' stones are regarded as a by-product of the gemstone market; they are used for abrasives). The cutting and polishing of rough diamonds is a specialized skill that is concentrated in a limited number of locations worldwide. Traditional diamond cutting centers are Antwerp, Amsterdam, Johannesburg, New York City, and Tel Aviv. Recently, diamond cutting centers have been established in China, India, Thailand, Namibia and Botswana. Cutting centers with lower cost of labor, notably Surat in Gujarat, Gujarat, India, handle a larger number of smaller carat diamonds, while smaller quantities of larger or more valuable diamonds are more likely to be handled in Europe or North America. The recent expansion of this industry in India, employing low cost labor, has allowed smaller diamonds to be prepared as gems in greater quantities than was previously economically feasible.
Diamonds prepared as gemstones are sold on diamond exchanges called ''Exchange (organized market), bourses''. There are 28 registered diamond bourses in the world. Bourses are the final tightly controlled step in the diamond supply chain; wholesalers and even retailers are able to buy relatively small lots of diamonds at the bourses, after which they are prepared for final sale to the consumer. Diamonds can be sold already set in jewelry, or sold unset ("loose"). According to the Rio Tinto, in 2002 the diamonds produced and released to the market were valued at US$9 billion as rough diamonds, US$14 billion after being cut and polished, US$28 billion in wholesale diamond jewelry, and US$57 billion in retail sales.
Further down the supply chain, members of The World Federation of Diamond Bourses (WFDB) act as a medium for wholesale diamond exchange, trading both polished and rough diamonds. The WFDB consists of independent diamond bourses in major cutting centers such as Tel Aviv, Antwerp, Johannesburg and other cities across the US, Europe and Asia. In 2000, the WFDB and The International Diamond Manufacturers Association established the World Diamond Council to prevent the trading of diamonds used to fund war and inhumane acts. WFDB's additional activities include sponsoring the World Diamond Congress every two years, as well as the establishment of the International Diamond Council (IDC) to oversee diamond grading.
Once purchased by Sightholders (which is a trademark term referring to the companies that have a three-year supply contract with DTC), diamonds are cut and polished in preparation for sale as gemstones ('industrial' stones are regarded as a by-product of the gemstone market; they are used for abrasives). The cutting and polishing of rough diamonds is a specialized skill that is concentrated in a limited number of locations worldwide. Traditional diamond cutting centers are Antwerp, Amsterdam, Johannesburg, New York City, and Tel Aviv. Recently, diamond cutting centers have been established in China, India, Thailand, Namibia and Botswana. Cutting centers with lower cost of labor, notably Surat in Gujarat, Gujarat, India, handle a larger number of smaller carat diamonds, while smaller quantities of larger or more valuable diamonds are more likely to be handled in Europe or North America. The recent expansion of this industry in India, employing low cost labor, has allowed smaller diamonds to be prepared as gems in greater quantities than was previously economically feasible.
Diamonds prepared as gemstones are sold on diamond exchanges called ''Exchange (organized market), bourses''. There are 28 registered diamond bourses in the world. Bourses are the final tightly controlled step in the diamond supply chain; wholesalers and even retailers are able to buy relatively small lots of diamonds at the bourses, after which they are prepared for final sale to the consumer. Diamonds can be sold already set in jewelry, or sold unset ("loose"). According to the Rio Tinto, in 2002 the diamonds produced and released to the market were valued at US$9 billion as rough diamonds, US$14 billion after being cut and polished, US$28 billion in wholesale diamond jewelry, and US$57 billion in retail sales.
 Marketing has significantly affected the image of diamond as a valuable commodity.
N. W. Ayer & Son, the advertising firm retained by De Beers in the mid-20th century, succeeded in reviving the American diamond market and the firm created new markets in countries where no diamond tradition had existed before. N. W. Ayer's marketing included product placement, advertising focused on the diamond product itself rather than the De Beers brand, and associations with celebrities and royalty. Without advertising the De Beers brand, De Beers was advertising its competitors' diamond products as well, but this was not a concern as De Beers dominated the diamond market throughout the 20th century. De Beers' market share dipped temporarily to second place in the global market below Alrosa in the aftermath of the global economic crisis of 2008, down to less than 29% in terms of carats mined, rather than sold. The campaign lasted for decades but was effectively discontinued by early 2011. De Beers still advertises diamonds, but the advertising now mostly promotes its own brands, or licensed product lines, rather than completely "generic" diamond products. The campaign was perhaps best captured by the slogan "a diamond is forever". This slogan is now being used by De Beers Diamond Jewelers, a jewelry firm which is a 50/50% joint venture between the De Beers mining company and LVMH, the luxury goods conglomerate.
Brown-colored diamonds constituted a significant part of the diamond production, and were predominantly used for industrial purposes. They were seen as worthless for jewelry (not even being assessed on the diamond color scale). After the development of Argyle diamond mine in Australia in 1986, and marketing, brown diamonds have become acceptable gems. The change was mostly due to the numbers: the Argyle mine, with its of diamonds per year, makes about one-third of global production of natural diamonds; 80% of Argyle diamonds are brown.
Marketing has significantly affected the image of diamond as a valuable commodity.
N. W. Ayer & Son, the advertising firm retained by De Beers in the mid-20th century, succeeded in reviving the American diamond market and the firm created new markets in countries where no diamond tradition had existed before. N. W. Ayer's marketing included product placement, advertising focused on the diamond product itself rather than the De Beers brand, and associations with celebrities and royalty. Without advertising the De Beers brand, De Beers was advertising its competitors' diamond products as well, but this was not a concern as De Beers dominated the diamond market throughout the 20th century. De Beers' market share dipped temporarily to second place in the global market below Alrosa in the aftermath of the global economic crisis of 2008, down to less than 29% in terms of carats mined, rather than sold. The campaign lasted for decades but was effectively discontinued by early 2011. De Beers still advertises diamonds, but the advertising now mostly promotes its own brands, or licensed product lines, rather than completely "generic" diamond products. The campaign was perhaps best captured by the slogan "a diamond is forever". This slogan is now being used by De Beers Diamond Jewelers, a jewelry firm which is a 50/50% joint venture between the De Beers mining company and LVMH, the luxury goods conglomerate.
Brown-colored diamonds constituted a significant part of the diamond production, and were predominantly used for industrial purposes. They were seen as worthless for jewelry (not even being assessed on the diamond color scale). After the development of Argyle diamond mine in Australia in 1986, and marketing, brown diamonds have become acceptable gems. The change was mostly due to the numbers: the Argyle mine, with its of diamonds per year, makes about one-third of global production of natural diamonds; 80% of Argyle diamonds are brown.


 Industrial diamonds are valued mostly for their hardness and thermal conductivity, making many of the gemological characteristics of diamonds, such as the 4 Cs, irrelevant for most applications. Eighty percent of mined diamonds (equal to about annually) are unsuitable for use as gemstones and are used industrially. In addition to mined diamonds, synthetic diamonds found industrial applications almost immediately after their invention in the 1950s; in 2014, of synthetic diamonds were produced, 90% of which were produced in China. Approximately 90% of diamond Grinding (abrasive cutting), grinding grit is currently of synthetic origin.
The boundary between gem-quality diamonds and industrial diamonds is poorly defined and partly depends on market conditions (for example, if demand for polished diamonds is high, some lower-grade stones will be polished into low-quality or small gemstones rather than being sold for industrial use). Within the category of industrial diamonds, there is a sub-category comprising the lowest-quality, mostly opaque stones, which are known as bort.
Industrial use of diamonds has historically been associated with their hardness, which makes diamond the ideal material for cutting and grinding tools. As the hardest known naturally occurring material, diamond can be used to polish, cut, or wear away any material, including other diamonds. Common industrial applications of this property include diamond-tipped drill bits and saws, and the use of diamond powder as an abrasive. Less expensive industrial-grade diamonds (bort) with more flaws and poorer color than gems, are used for such purposes. Diamond is not suitable for machining ferrous alloys at high speeds, as carbon is soluble in iron at the high temperatures created by high-speed machining, leading to greatly increased wear on diamond tools compared to alternatives.
Specialized applications include use in laboratories as containment for Pressure experiment, high-pressure experiments (see diamond anvil cell), high-performance bearing (mechanical), bearings, and limited use in specialized windows. With the continuing advances being made in the production of synthetic diamonds, future applications are becoming feasible. The high
Industrial diamonds are valued mostly for their hardness and thermal conductivity, making many of the gemological characteristics of diamonds, such as the 4 Cs, irrelevant for most applications. Eighty percent of mined diamonds (equal to about annually) are unsuitable for use as gemstones and are used industrially. In addition to mined diamonds, synthetic diamonds found industrial applications almost immediately after their invention in the 1950s; in 2014, of synthetic diamonds were produced, 90% of which were produced in China. Approximately 90% of diamond Grinding (abrasive cutting), grinding grit is currently of synthetic origin.
The boundary between gem-quality diamonds and industrial diamonds is poorly defined and partly depends on market conditions (for example, if demand for polished diamonds is high, some lower-grade stones will be polished into low-quality or small gemstones rather than being sold for industrial use). Within the category of industrial diamonds, there is a sub-category comprising the lowest-quality, mostly opaque stones, which are known as bort.
Industrial use of diamonds has historically been associated with their hardness, which makes diamond the ideal material for cutting and grinding tools. As the hardest known naturally occurring material, diamond can be used to polish, cut, or wear away any material, including other diamonds. Common industrial applications of this property include diamond-tipped drill bits and saws, and the use of diamond powder as an abrasive. Less expensive industrial-grade diamonds (bort) with more flaws and poorer color than gems, are used for such purposes. Diamond is not suitable for machining ferrous alloys at high speeds, as carbon is soluble in iron at the high temperatures created by high-speed machining, leading to greatly increased wear on diamond tools compared to alternatives.
Specialized applications include use in laboratories as containment for Pressure experiment, high-pressure experiments (see diamond anvil cell), high-performance bearing (mechanical), bearings, and limited use in specialized windows. With the continuing advances being made in the production of synthetic diamonds, future applications are becoming feasible. The high  Historically, diamonds were found only in
Historically, diamonds were found only in