Desymmetrization on:
[Wikipedia]
[Google]
[Amazon]
Desymmetrization is a chemical reaction that converts
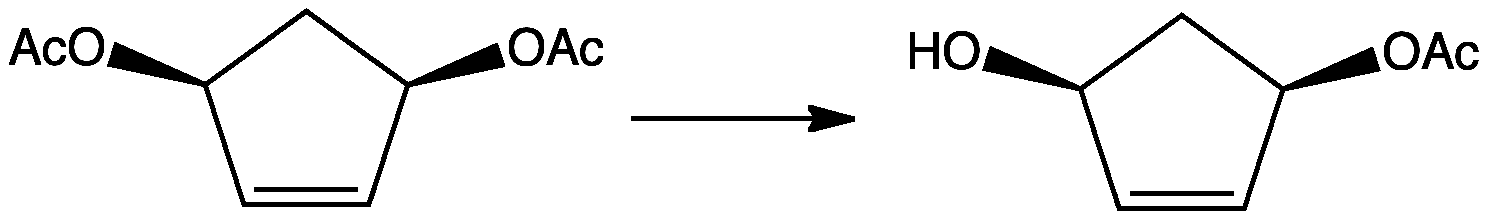 In another example, a symmetrical cyclic
In another example, a symmetrical cyclic  The alcoholysis of cyclic anhydrides can be conducted enantiosymmetrically using chiral amine catalysts.
A related example is the hydrolysis of prochiral diesters catalyzed by chiral
The alcoholysis of cyclic anhydrides can be conducted enantiosymmetrically using chiral amine catalysts.
A related example is the hydrolysis of prochiral diesters catalyzed by chiral
prochiral
In stereochemistry, prochiral molecules are those that can be converted from achiral to chiral in a single step, such as changing one atom. An achiral species which can be converted to a chiral in two steps is called proprochiral.
A molecule ha ...
substrates into chiral products. Desymmetrisations are so pervasive that they are rarely described as such except when they proceed ''enantioselectively''. The enantioselective reactions require chiral catalysts or chiral reagents.Willis, Michael C. "Enantioselective desymmetrization" Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry 1999, pp. 1765-1784. According to IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
, desymmetrization involves the "... the conversion of a prochiral molecular entity into a chiral one."
Examples
Typical substrates areepoxide
In organic chemistry, an epoxide is a cyclic ether, where the ether forms a three-atom ring: two atoms of carbon and one atom of oxygen. This triangular structure has substantial ring strain, making epoxides highly reactive, more so than other ...
s, diol
A diol is a chemical compound containing two hydroxyl groups ( groups). An aliphatic diol may also be called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified. They are used as protecting gro ...
s, diene
In organic chemistry, a diene ( ); also diolefin, ) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alk''ene'' units, with the standard prefix ''di'' of systematic nome ...
s, and cyclic carboxylic acid anhydride
An organic acid anhydride is an acid anhydride that is also an organic compound. An acid anhydride is a compound that has two acyl groups chemical bond, bonded to the same oxygen atom. A common type of organic acid anhydride is a carboxylic anhyd ...
s.
One example is the conversion of cis-3,5-diacetoxycyclopentene to monoacetate. This particular conversion utilizes the enzyme cholinesterase
The enzyme cholinesterase (EC 3.1.1.8, choline esterase; systematic name acylcholine acylhydrolase) catalyses the hydrolysis of choline-based esters:
: an acylcholine + H2O = choline + a carboxylate
Several of these serve as neurotransmitte ...
.
: In another example, a symmetrical cyclic
In another example, a symmetrical cyclic imide
In organic chemistry, an imide is a functional group consisting of two acyl groups bound to nitrogen. The compounds are structurally related to acid anhydrides, although imides are more resistant to hydrolysis. In terms of commercial applications ...
is subjected to asymmetric deprotonation resulting in a chiral product with high enantioselectivity.
Partial hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated ...
converts benzil
Benzil (i.e. Bz2, systematically known as 1,2-diphenylethane-1,2-dione) is the organic compound with the formula ( C6H5 CO)2, generally abbreviated ( PhCO)2. This yellow solid is one of the most common diketones. Its main use is as a photoinitiat ...
(PhC(O)C(O)Ph) into chiral hydro benzoin. The process can be implemented enantioselectively using transfer hydrogenation
In chemistry, transfer hydrogenation is a chemical reaction involving the addition of hydrogen to a compound from a source other than molecular . It is applied in laboratory and industrial organic synthesis to saturate organic compounds and re ...
.
: (Ph =
The precursor benzil has C2v symmetry
Symmetry () in everyday life refers to a sense of harmonious and beautiful proportion and balance. In mathematics, the term has a more precise definition and is usually used to refer to an object that is Invariant (mathematics), invariant und ...
, and the product is C2 symmetric.
Citric acid is also a symmetric molecule that can be desymmetrized by partial methylation.
phosphoric acid
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula . It is commonly encountered as an 85% aqueous solution, ...
s.
Formal symmetry considerations
Desymmetrizations involve the loss of an improper axis of rotation (mirror plane, center of inversion, rotation-reflection axis). In other words, desymmetrisations convertprochiral
In stereochemistry, prochiral molecules are those that can be converted from achiral to chiral in a single step, such as changing one atom. An achiral species which can be converted to a chiral in two steps is called proprochiral.
A molecule ha ...
precursors into chiral
Chirality () is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek language, Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is dist ...
products.''Basic Terminology of Stereochemistry'', G.P. Moss Ed. Pure Appl. Chem., Vol. 68, No. 12, pp. 2193-2222, 1996.
References
Stereochemistry {{Stereochemistry-stub