Death Effector Domain on:
[Wikipedia]
[Google]
[Amazon]
The death-effector domain (DED) is a protein interaction domain found only in eukaryotes that regulates a variety of cellular signalling pathways. The DED domain is found in inactive
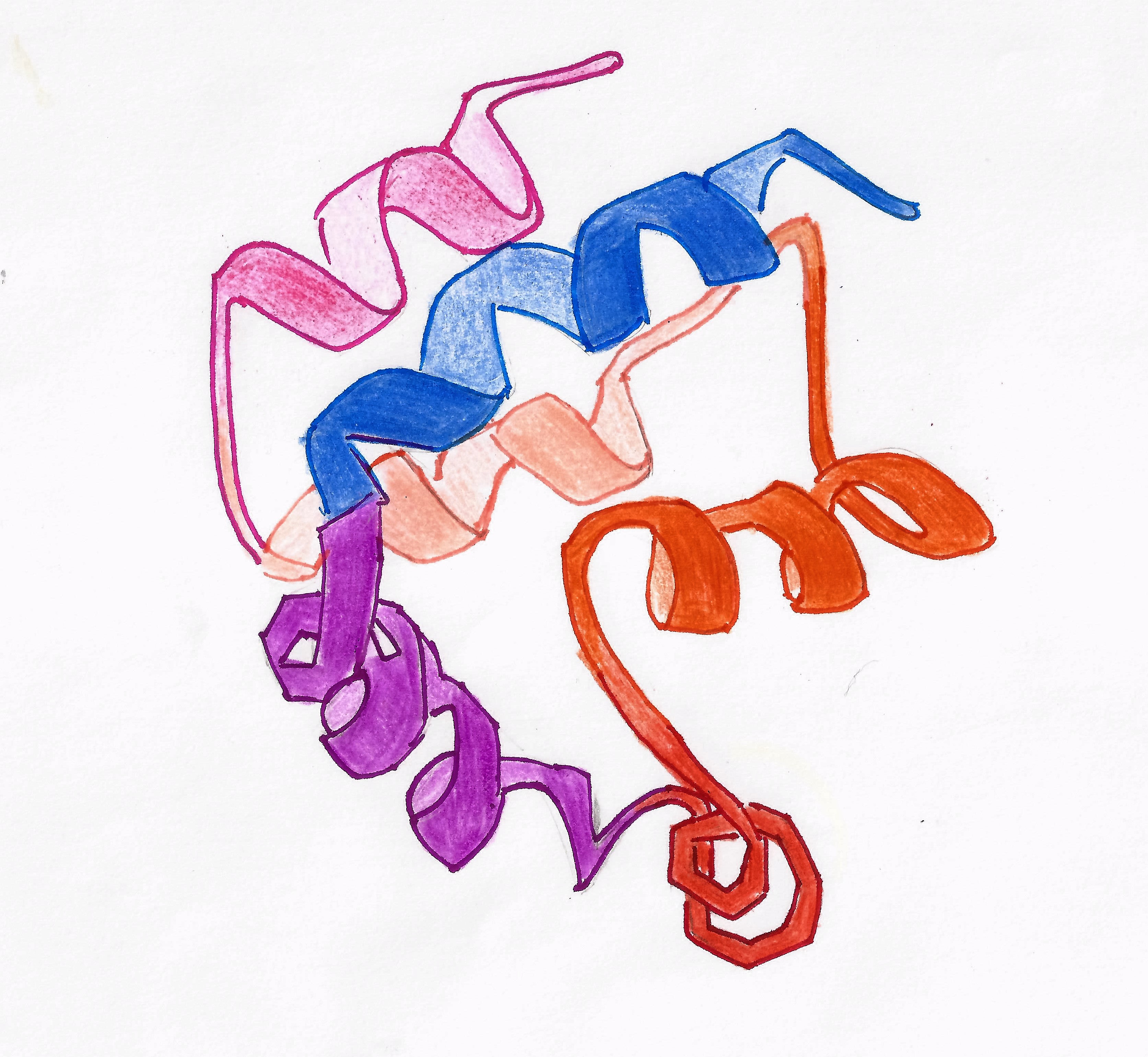 DED is a subfamily of the DD superfamily (other recognizable domains in this superfamily are: caspase-recruitment domain (CARD), pyrin domain (PYD) and death domain (DD)). The subfamilies resemble structurally one another, all of them (and DED in particular) are composed of a bundle of 6 alpha-helices, but they diverge in the surface features.
The complete primary structure of this proteic domain has not been consensually defined. Some studies described residues 2-184, but C-terminus and
DED is a subfamily of the DD superfamily (other recognizable domains in this superfamily are: caspase-recruitment domain (CARD), pyrin domain (PYD) and death domain (DD)). The subfamilies resemble structurally one another, all of them (and DED in particular) are composed of a bundle of 6 alpha-helices, but they diverge in the surface features.
The complete primary structure of this proteic domain has not been consensually defined. Some studies described residues 2-184, but C-terminus and
 These interactions are defined by helices α1/α4 and α2/α3: residues Ser1, Val6, His9, Leu43, Asp44 and Glu51 from α1/α4 are in contact with Thr21, Phe25, Lys33, Arg34, Glu37 and Glu51 from α2/α3 of the second molecule. Each interaction involves an area of 1062 Å2 and contributions from hydrophobic side chains, hydrogen bonding and salt bridges. The final homodimer has a structure oriented so that each subunit has the 2 interaction sites.
Procaspase-8, also a DED-containing protein, has affinity for the FADD DED. It's for that reason that they are recruited to FADD as monomers via their DEDs. These interaction is defined by α1/α4 of procapase-8 DED-A and FADD DED α2/α3 or α1/α4 of FADD DED and α2/α5 of procapase-8 DED-B. Procaspase-8 DED-B interacts with FADD and DED-A mediates capase-8 chain formation, or vice versa.
However, in both cases the interaction leads to create a dimer between procaspases, which generates a conformational change. This dimerization is essential to create the active site; a p12 subunit is liberated and it is subsequently processed to the small p10 subunit. The two molecules of procapase-8 are associated with these p10 subunits creating an active protease-8 cell death.
These interactions are defined by helices α1/α4 and α2/α3: residues Ser1, Val6, His9, Leu43, Asp44 and Glu51 from α1/α4 are in contact with Thr21, Phe25, Lys33, Arg34, Glu37 and Glu51 from α2/α3 of the second molecule. Each interaction involves an area of 1062 Å2 and contributions from hydrophobic side chains, hydrogen bonding and salt bridges. The final homodimer has a structure oriented so that each subunit has the 2 interaction sites.
Procaspase-8, also a DED-containing protein, has affinity for the FADD DED. It's for that reason that they are recruited to FADD as monomers via their DEDs. These interaction is defined by α1/α4 of procapase-8 DED-A and FADD DED α2/α3 or α1/α4 of FADD DED and α2/α5 of procapase-8 DED-B. Procaspase-8 DED-B interacts with FADD and DED-A mediates capase-8 chain formation, or vice versa.
However, in both cases the interaction leads to create a dimer between procaspases, which generates a conformational change. This dimerization is essential to create the active site; a p12 subunit is liberated and it is subsequently processed to the small p10 subunit. The two molecules of procapase-8 are associated with these p10 subunits creating an active protease-8 cell death.

 This is how DED can also inhibit the apoptosis cascade, and the consequence is necroptosis.
This is how DED can also inhibit the apoptosis cascade, and the consequence is necroptosis.
The Nash Lab: DED
InterPro: Death effector domainSMART: DED
{{Protein domains Protein domains
procaspases
Caspases (cysteine-aspartic proteases, cysteine aspartases or cysteine-dependent aspartate-directed proteases) are a family of protease enzymes playing essential roles in programmed cell death. They are named caspases due to their specific cy ...
(cysteine protease
Cysteine proteases, also known as thiol proteases, are hydrolase enzymes that degrade proteins. These proteases share a common catalytic mechanism that involves a nucleophilic cysteine thiol in a catalytic triad or dyad.
Discovered by Gopal ...
s) and proteins that regulate caspase activation in the apoptosis cascade such as FAS-associating death domain-containing protein
FAS-associated death domain protein, also called MORT1, is encoded by the ''FADD'' gene on the 11q13.3 region of chromosome 11 in humans.
FADD is an adaptor protein that bridges members of the tumor necrosis factor receptor superfamily, such ...
(FADD
FAS-associated death domain protein, also called MORT1, is encoded by the ''FADD'' gene on the 11q13.3 region of chromosome 11 in humans.
FADD is an adaptor protein that bridges members of the tumor necrosis factor receptor superfamily, such ...
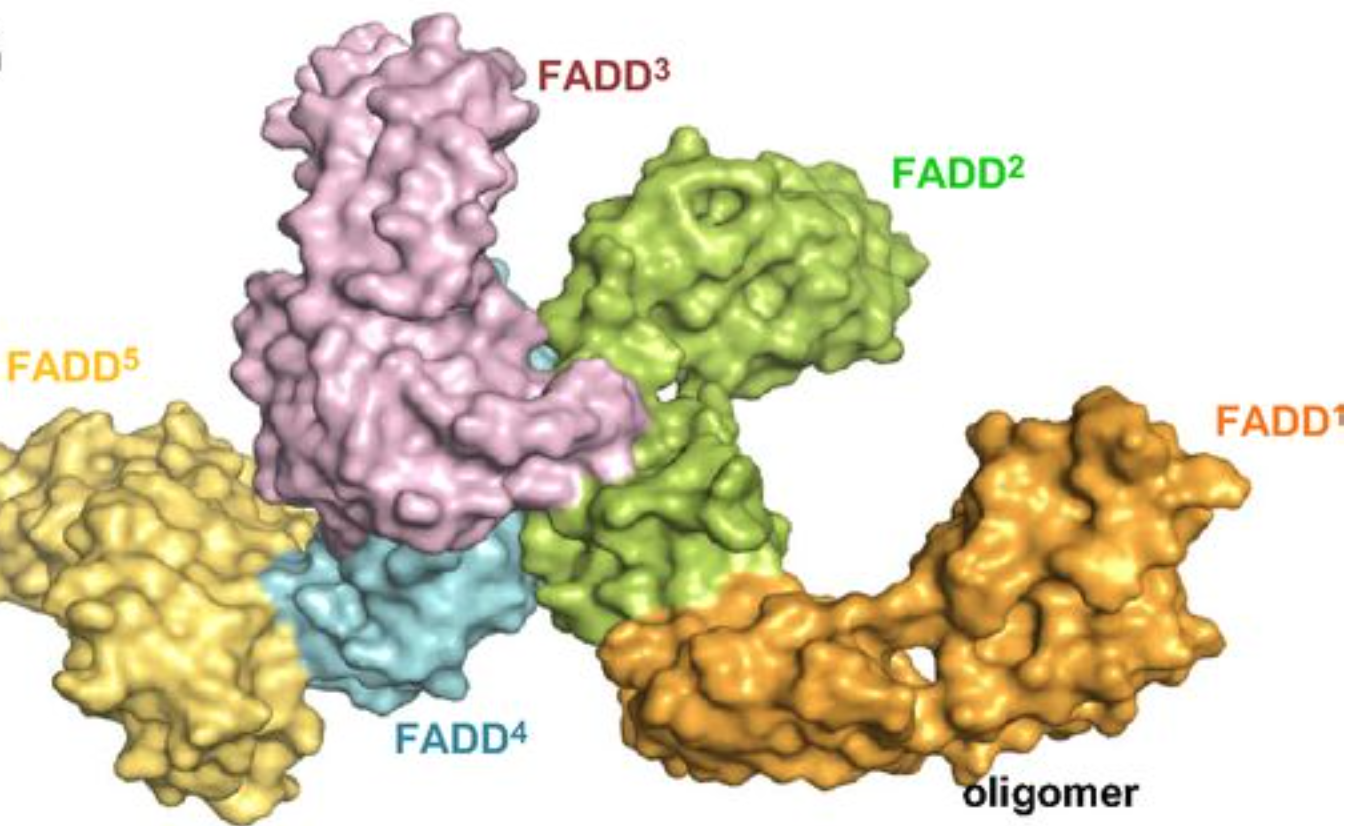
). FADD recruits procaspase 8 and procaspase 10 into a death induced signaling complex (DISC). This recruitment is mediated by a homotypic interaction between the procaspase DED and a second DED that is death effector domain in an adaptor protein that is directly associated with activated TNF receptors. Complex formation allows proteolytic activation of procaspase into the active caspase form which results in the initiation of apoptosis (cell death). Structurally the DED domain are a subclass of protein motif known as the death fold and contains 6 alpha helices, that closely resemble the structure of the Death domain
Death is the irreversible cessation of all biological functions that sustain an organism. For organisms with a brain, death can also be defined as the irreversible cessation of functioning of the whole brain, including brainstem, and brain ...
(DD).
Structure
 DED is a subfamily of the DD superfamily (other recognizable domains in this superfamily are: caspase-recruitment domain (CARD), pyrin domain (PYD) and death domain (DD)). The subfamilies resemble structurally one another, all of them (and DED in particular) are composed of a bundle of 6 alpha-helices, but they diverge in the surface features.
The complete primary structure of this proteic domain has not been consensually defined. Some studies described residues 2-184, but C-terminus and
DED is a subfamily of the DD superfamily (other recognizable domains in this superfamily are: caspase-recruitment domain (CARD), pyrin domain (PYD) and death domain (DD)). The subfamilies resemble structurally one another, all of them (and DED in particular) are composed of a bundle of 6 alpha-helices, but they diverge in the surface features.
The complete primary structure of this proteic domain has not been consensually defined. Some studies described residues 2-184, but C-terminus and N-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the ami ...
residues are not identified yet. The presence of amino acids that determine the solubility and aggregation to DED allowed to identify DED's in different proteins, such as caspase-8 and MC159. The secondary structure of the domain, as said, is built by 6 alpha-helices.
The tertiary structure of the domain has been described from the crystallization
Crystallization is the process by which solid forms, where the atoms or molecules are highly organized into a structure known as a crystal. Some ways by which crystals form are precipitating from a solution, freezing, or more rarely de ...
of caspase 8
Caspase-8 is a caspase protein, encoded by the ''CASP8'' gene. It most likely acts upon caspase-3.
''CASP8'' orthologs have been identified in numerous mammals for which complete genome data are available. These unique orthologs are also prese ...
in the human. The method used to describe the structure was X-RAY diffraction and the resolution obtained is 2.2 Å. DEDs in this protein show an asymmetric unit dimer, with its interface contains two hydrogen bonding networks, that appear as a filamentous structure.
DED's function is determined by its structure. As far as it is known, the homotypic interactions that activate caspase and trigger apoptosis are mediated by asymmetrical surface contacts between partners (like DED1 and DED2 in the caspase-8 case). The residues that form the surfaces are typically charged amino acids, but a short hydrophobic patch can also be observed on the interactive surface of the domain.
Function
DED domain is best known for its role in apoptosis. However, DED-containing proteins are also involved in other cellular processes so that they control both life and death cell decisions.Extrinsic apoptosis
Apoptosis is a controlled and programmed cell death that confers advantages during an organism lifecycle. The extrinsic pathway is directed by a family of proteases which become active in response to death stimuli. To know the role of DEDs in this process is important to observe the formation of the multiprotein death-including signalling complex (DISC). DR4,TRAIL-R2
Death receptor 5 (DR5), also known as TRAIL receptor 2 (TRAILR2) and tumor necrosis factor receptor superfamily member 10B (TNFRSF10B), is a cell surface receptor of the TNF-receptor superfamily that binds TRAIL and mediates apoptosis.
Function ...
and CD95 are death receptors (members of TNF receptor superfamily
The tumor necrosis factor receptor superfamily (TNFRSF) is a protein superfamily of cytokine receptors characterized by the ability to bind tumor necrosis factors (TNFs) via an extracellular cysteine-rich domain. With the exception of nerve growth ...
) which interact together using their intracellular death domain
Death is the irreversible cessation of all biological functions that sustain an organism. For organisms with a brain, death can also be defined as the irreversible cessation of functioning of the whole brain, including brainstem, and brain ...
s (DDs). The DD of FADD, a protein containing a DED, can then interact with these DDs described. Here the function of FADD DED is to create a stabilized structure by self-associating FADD. These interactions are defined by helices α1/α4 and α2/α3: residues Ser1, Val6, His9, Leu43, Asp44 and Glu51 from α1/α4 are in contact with Thr21, Phe25, Lys33, Arg34, Glu37 and Glu51 from α2/α3 of the second molecule. Each interaction involves an area of 1062 Å2 and contributions from hydrophobic side chains, hydrogen bonding and salt bridges. The final homodimer has a structure oriented so that each subunit has the 2 interaction sites.
Procaspase-8, also a DED-containing protein, has affinity for the FADD DED. It's for that reason that they are recruited to FADD as monomers via their DEDs. These interaction is defined by α1/α4 of procapase-8 DED-A and FADD DED α2/α3 or α1/α4 of FADD DED and α2/α5 of procapase-8 DED-B. Procaspase-8 DED-B interacts with FADD and DED-A mediates capase-8 chain formation, or vice versa.
However, in both cases the interaction leads to create a dimer between procaspases, which generates a conformational change. This dimerization is essential to create the active site; a p12 subunit is liberated and it is subsequently processed to the small p10 subunit. The two molecules of procapase-8 are associated with these p10 subunits creating an active protease-8 cell death.
These interactions are defined by helices α1/α4 and α2/α3: residues Ser1, Val6, His9, Leu43, Asp44 and Glu51 from α1/α4 are in contact with Thr21, Phe25, Lys33, Arg34, Glu37 and Glu51 from α2/α3 of the second molecule. Each interaction involves an area of 1062 Å2 and contributions from hydrophobic side chains, hydrogen bonding and salt bridges. The final homodimer has a structure oriented so that each subunit has the 2 interaction sites.
Procaspase-8, also a DED-containing protein, has affinity for the FADD DED. It's for that reason that they are recruited to FADD as monomers via their DEDs. These interaction is defined by α1/α4 of procapase-8 DED-A and FADD DED α2/α3 or α1/α4 of FADD DED and α2/α5 of procapase-8 DED-B. Procaspase-8 DED-B interacts with FADD and DED-A mediates capase-8 chain formation, or vice versa.
However, in both cases the interaction leads to create a dimer between procaspases, which generates a conformational change. This dimerization is essential to create the active site; a p12 subunit is liberated and it is subsequently processed to the small p10 subunit. The two molecules of procapase-8 are associated with these p10 subunits creating an active protease-8 cell death.

Necroptosis
During the creation of the DISC procaspase-8 can also heterodimerise with another DED-containing protein known as FLIPL. FLIPL’s pseudo-caspase has two tandem DEDs that are very similar to the N-terminus of capase-8, but in which there is an important mutation in the active site (cysteine to tyrosine). This heterodimeration done between their DEDs prevents from the normal homodimeration so that the pseudo-caspase is unable to activate the apoptotic cascade. FLIPL ’s pseudo-caspase is more efficient at inducing the conformational change. However, FLIPL hasn’t enough enzymatic activity so that cleavage between the DEDs and p18 it’s not possible. In consequence it’s impossible to create the active protease cell death. Procaspase-8 can also heterodimerise with FLIPS, also a DED containing protein. In this case heterodimerisation directly fails to activate procaspase-8 as the initial conformational change cannot take place in procaspase-8’s caspase domain. This is how DED can also inhibit the apoptosis cascade, and the consequence is necroptosis.
This is how DED can also inhibit the apoptosis cascade, and the consequence is necroptosis.
The DED protein family
DED-containing proteins
Caspase-8 and caspase-10
Caspases arecysteine protease
Cysteine proteases, also known as thiol proteases, are hydrolase enzymes that degrade proteins. These proteases share a common catalytic mechanism that involves a nucleophilic cysteine thiol in a catalytic triad or dyad.
Discovered by Gopal ...
s responsible for dismantling off the cell during apoptosis.
These proteins are zymogens and become active after their cleavage at specific sites within the molecule.
Structure:
* Death Effector Domain (DED) and a Caspase Recruitment Domain (CARD) that are englobed in a structure called pro-domain, which is located at the N-terminus
* Catalytic protease domain at the C-terminus.
There are two groups of protease
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalyzes (increases reaction rate or "speeds up") proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the form ...
s:
* Effector caspases: induce the biggest part of the morphological changes that occur during apoptosis.
* Initiator caspases: responsible for the activation of effector caspases. These caspases are activated through oligomer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relat ...
ization and cleavage that make the protein functional.
The two tandem DEDs in the pro-domain of caspase induce the protein-protein interactions with other proteins like the FADD
FAS-associated death domain protein, also called MORT1, is encoded by the ''FADD'' gene on the 11q13.3 region of chromosome 11 in humans.
FADD is an adaptor protein that bridges members of the tumor necrosis factor receptor superfamily, such ...
.
Studying caspases is important since they don’t only control apoptosis but also inhibit it, depending on the necessity of the cell. Scientists find that they are a mechanism that can regulate cell life and is important for cancer therapies.
FLICE-like inhibitory proteins (FLIPs)
FLICE-like inhibitory proteins (FLIPs) are cell inhibitors capable of stopping the death receptors’ signal, which cause cell apoptosis. The first FLIPs that were identified were expressed by γ-herpes viruses so they were called v-FLIPs. These v-FLIPs were able to associate with the receptor in the death-inducing signaling complex (DISC), blocking that way the CD95-mediated apoptosis. vFLIPs predominantly contain two sequential DEDs, which are highly homologous to the N-terminus of caspase-8. The cellular homologues of v-FLIPs are generally expressed in two forms: * c-FLIPS (short): it contains only the amino-terminal tandem DEDs followed by a short carboxy-terminal section. Its structure is similar to the viral FLIPs. * c-FLIPL (long): it consists of not only the tandem DEDs, but also a protease-like domain (homologous to caspase-8) in which different important for protease activity amino acids are mutated, including the active site cysteine. Both forms of c-FLIP are draft to the CD95 DISC, where they heterodimerize with caspase-8. c-FLIP has been involved in signaling alternative pathways, connecting the CD95 receptor to theNF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) is a protein complex that controls transcription of DNA, cytokine production and cell survival. NF-κB is found in almost all animal cell types and is involved in cellular ...
, JNK and MAPK
A mitogen-activated protein kinase (MAPK or MAP kinase) is a type of protein kinase that is specific to the amino acids serine and threonine (i.e., a serine/threonine-specific protein kinase). MAPKs are involved in directing cellular responses ...
pathways.
PEA-15/PED
PEA-15 (Phosphoprotein Enriched in Astrocytes-15 kDa) also known as PED (Phosphoprotein Enriched in Diabetes) is a DED-containing protein with pleiotropic effects. PED is a small, non-catalytic, protein consisting of an N-terminal death-effector domain (DED) and a C-terminal tail with irregular structure. PED/PEA-15 interacts with various types of proteins with and without DEDs, and its specificity of joining these proteins is mediated by thephosphorylation
In chemistry, phosphorylation is the attachment of a phosphate group to a molecule or an ion. This process and its inverse, dephosphorylation, are common in biology and could be driven by natural selection. Text was copied from this source, ...
on two serine residues on the C-terminal tail:
* Ser104: phosphorylated by protein kinase C (PKC).
* Ser116: the substrate for calcium/calmodulin-dependent protein kinase II (CamKII).
PEA-15 works as an antiapoptotic DED protein in several signaling cascades. In TNF α-, CD95- and TRAIL-mediated pathways, PEA-15 acts binding and disrupting FADD and caspase-8 interactions.
Besides apoptosis, PEA-15 inhibits the insulin-mediated glucose transport in muscle cells, so a high level expression of PEA-15's mRNA has been associated to diabetes mellitus type II
Diabetes, also known as diabetes mellitus, is a group of metabolic disorders characterized by a high blood sugar level (hyperglycemia) over a prolonged period of time. Symptoms often include frequent urination, increased thirst and increased ...
.
DEDD/DEDD2
Death effector domain containing DNA binding (DEDD). Shows DNA binding capacity, localized in the nucleoli in overexpression where it associates with a molecule called DEDAF (DED-associated factor) that potentiates apoptosis. In addition it blocksRNA polymerase I
RNA polymerase 1 (also known as Pol I) is, in higher eukaryotes, the polymerase that only transcribes ribosomal RNA (but not 5S rRNA, which is synthesized by RNA polymerase III), a type of RNA that accounts for over 50% of the total RNA synthesize ...
transcription by binding to the DNA.
DEDD2 (FLAME-3) is a DEDD homologue that shares a 48.5% of the amino acidic sequence. It is noted to interact with c-FLIP and DEDD and to have an important role in polymerase II-dependent transcription repression.
Proteins with a DED-related domain
HIP-1 and HIPPI
Huntingtin interacting protein-1 (HIP-1) is a protein that interacts with huntingtin (Htt), another protein that when is mutated (with expanded polyglutamine repeats) forms protein aggregates in the brain of patients with Huntington's disease (HD). HIP-1 contains a pseudo death effector domain (pDED), that's why the overexpression of HIP-1 induces apoptosis in several cells as DED proteins do. This type of apoptosis depends on the pDED of the HIP-1, and it consists in the activation of caspase-3, an enzyme that is reduced when wild-type Htt is expressed, that fact suggests that HIP-1 cooperates with Htt in the pathomechanism of Huntington's disease. By yeasttwo-hybrid screening
Two-hybrid screening (originally known as yeast two-hybrid system or Y2H) is a molecular biology technique used to discover protein–protein interactions (PPIs) and protein–DNA interactions by testing for physical interactions (such as bind ...
, HIP-1 has shown to interact with a protein of 419 amino acids called HIPPI (HIP-1 protein interactor). Succeeding experiments have revealed that the presence of HIPPI determines the HIP-1-induced apoptosis.
FLASH
FLICE-associated huge protein. Contains a similar domain to DED, but the homology is very weak and its function is still unclear.Therapeutically exploiting DED
DED complexes have been shown to function at crucial steps controlling life and death cell processes. This knowledge is particularly useful in therapy because there are so many pathologies related with an abnormal control of the cell life. The absence of apoptosis is feature of cancer. In some cases the gene encoding procaspase-8 is silenced by methylation, so it is necessary to activate the gene using epigenetic treatments to have active protease. In other cases there is an overexpression of FLIP, the anti-apoptotic molecule that prevents from the formation of the active caspase. In this case there are some anti-cancer agents that downregulate FLIP expression. However, the abnormal apoptosis it is not exclusive from cancer, there are other pathologies such as inflammation and neurodegenerative diseases than can also be treated with these kind of therapeutics.References
External links
The Nash Lab: DED
InterPro: Death effector domain
{{Protein domains Protein domains