DD-transpeptidase on:
[Wikipedia]
[Google]
[Amazon]
DD-Transpeptidase (, ''DD-peptidase'', ''DD-transpeptidase'', ''DD-carboxypeptidase'', ''D-alanyl-D-alanine
 Crosslinking of peptidyl moieties of adjacent
Crosslinking of peptidyl moieties of adjacent
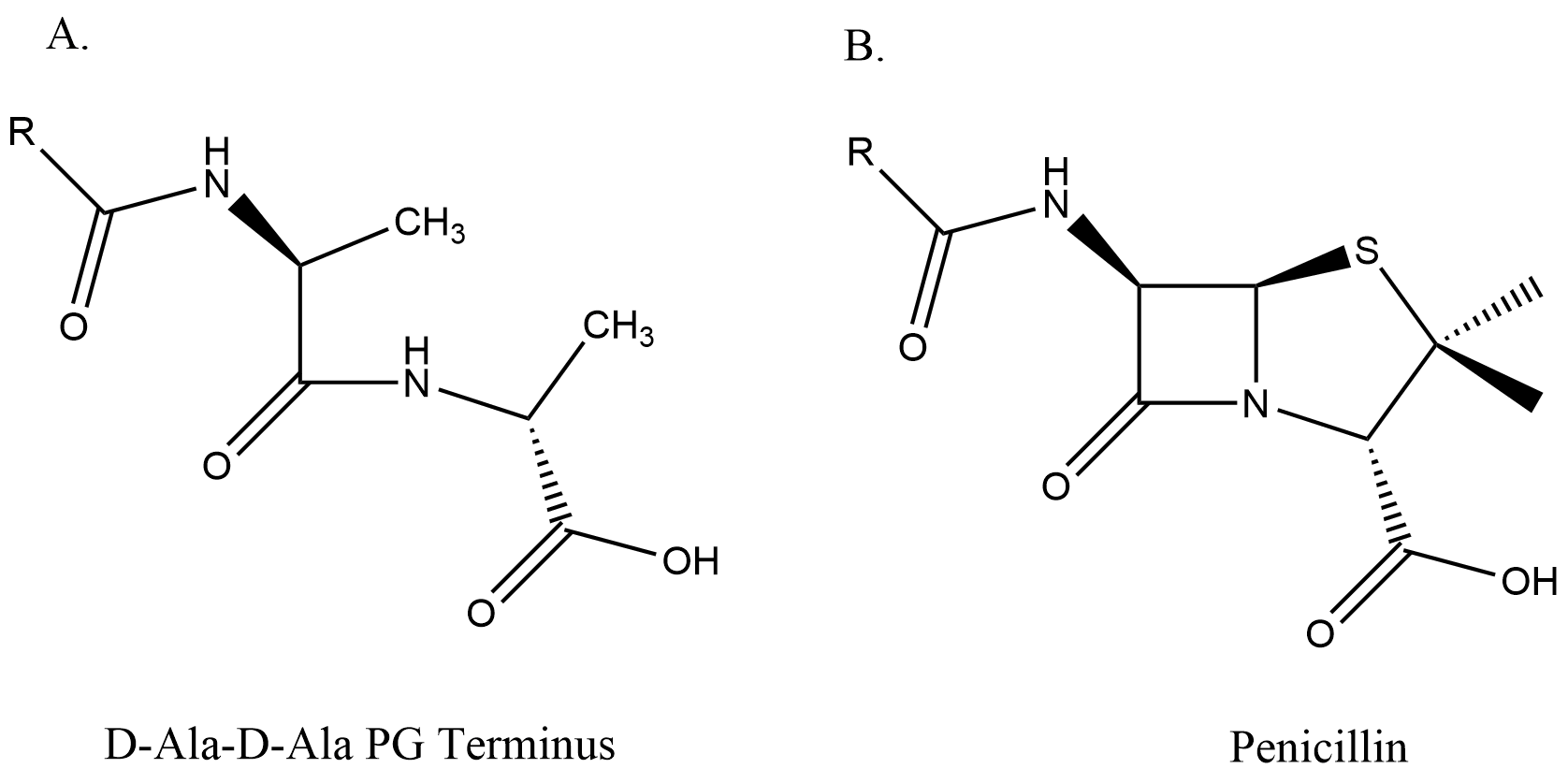 This enzyme is an excellent drug target because it is essential, is accessible from the
This enzyme is an excellent drug target because it is essential, is accessible from the
S11.001
* * {{Portal bar, Biology, border=no EC 3.4.16 Bacteria Microbial metabolism
carboxypeptidase
A carboxypeptidase ( EC number 3.4.16 - 3.4.18) is a protease enzyme that hydrolyzes (cleaves) a peptide bond at the carboxy-terminal (C-terminal) end of a protein or peptide. This is in contrast to an aminopeptidases, which cleave peptide b ...
'', ''D-alanyl-D-alanine-cleaving-peptidase'', ''D-alanine carboxypeptidase'', ''D-alanyl carboxypeptidase'', and ''serine-type D-Ala-D-Ala carboxypeptidase''.) is a bacterial enzyme that catalyzes the transfer of the R-L-αα-D-alanyl moiety of R-L-αα-D-alanyl-D-alanine carbonyl donors to the γ-OH of their active-site serine and from this to a final acceptor. It is involved in bacterial cell wall biosynthesis, namely, the transpeptidation that crosslinks the peptide side chains of peptidoglycan strands.
The antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting pathogenic bacteria, bacterial infections, and antibiotic medications are widely used in the therapy ...
penicillin
Penicillins (P, PCN or PEN) are a group of beta-lactam antibiotic, β-lactam antibiotics originally obtained from ''Penicillium'' Mold (fungus), moulds, principally ''Penicillium chrysogenum, P. chrysogenum'' and ''Penicillium rubens, P. ru ...
irreversibly binds to and inhibits the activity of the transpeptidase enzyme by forming a highly stable penicilloyl-enzyme intermediate. Because of the interaction between penicillin and transpeptidase, this enzyme is also known as penicillin-binding protein (PBP).
Mechanism
DD-Transpeptidase is mechanistically similar to the proteolytic reactions of the trypsin protein family. Crosslinking of peptidyl moieties of adjacent
Crosslinking of peptidyl moieties of adjacent glycan
The terms glycans and polysaccharides are defined by IUPAC as synonyms meaning "compounds consisting of a large number of monosaccharides linked glycosidically". However, in practice the term glycan may also be used to refer to the carbohydrate ...
strands is a two-step reaction. The first step involves the cleavage of the D-alanyl-D-alanine bond of a peptide unit precursor acting as carbonyl donor, the release of the carboxyl-terminal D-alanine, and the formation of the acyl-enzyme. The second step involves the breakdown of the acyl-enzyme intermediate and the formation of a new peptide bond between the carbonyl of the D-alanyl moiety and the amino group of another peptide unit.
Most discussion of DD-peptidase mechanisms revolves around the catalysts of proton transfer. During formation of the acyl-enzyme intermediate, a proton must be removed from the active site serine hydroxyl group and one must be added to the amine leaving group. A similar proton movement must be facilitated in deacylation. The identity of the general acid and base catalysts involved in these proton transfers has not yet been elucidated. However, the catalytic triad tyrosine, lysine, and serine, as well as serine, lysine, serine have been proposed.
Structure
Transpeptidases are members of the penicilloyl-serine transferase superfamily, which has a signature SxxK conserved motif. With "x" denoting a variableamino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
residue, the transpeptidases of this superfamily show a trend in the form of three motifs: SxxK, SxN (or analogue), and KTG (or analogue). These motifs occur at equivalent places, and are roughly equally spaced, along the polypeptide chain. The folded protein brings these motifs close to each other at the catalytic center between an all-α domain and an α/β domain.
The structure of the streptomyces K15 DD-transpeptidase has been studied, and consists of a single polypeptide chain organized into two domains. One domain contains mainly α-helices, and the second one is of α/β-type. The center of the catalytic cleft is occupied by the Ser35-Thr36-Thr37-Lys38 tetrad, which includes the nucleophilic Ser35 residue at the amino-terminal end of helix α2. One side of the cavity is defined by the Ser96-Gly97-Cys98 loop connecting helices α4 and α5. The Lys213-Thr214-Gly215 triad lies on strand β3 on the opposite side of the cavity. The backbone NH group of the essential Ser35 residue and that of Ser216 downstream from the motif Lys213-Thr214-Gly215 occupy positions that are compatible with the oxyanion hole
An oxyanion hole is a pocket in the active site of an enzyme that stabilizes transition state negative charge on a deprotonation, deprotonated oxygen or alkoxide. The pocket typically consists of backbone amides or positively charged residues. Sta ...
function required for catalysis.
The enzyme is classified as a DD-transpeptidase because the susceptible peptide bond of the carbonyl donor extends between two carbon atoms with the D-configuration.
Biological Function
All bacteria possess at least one, most often several, monofunctional serine DD-peptidases.Disease Relevance
 This enzyme is an excellent drug target because it is essential, is accessible from the
This enzyme is an excellent drug target because it is essential, is accessible from the periplasm
The periplasm is a concentrated gel-like matrix in the space between the inner cytoplasmic membrane and the bacterial outer membrane called the ''periplasmic space'' in Gram-negative (more accurately "diderm") bacteria. Using cryo-electron micros ...
, and has no equivalent in mammalian cells. DD-Transpeptidase is the target protein of β-lactam antibiotics (e.g. penicillin
Penicillins (P, PCN or PEN) are a group of beta-lactam antibiotic, β-lactam antibiotics originally obtained from ''Penicillium'' Mold (fungus), moulds, principally ''Penicillium chrysogenum, P. chrysogenum'' and ''Penicillium rubens, P. ru ...
). This is because the structure of the β-lactam closely resembles the D-ala-D-ala residue.
β-Lactams exert their effect by competitively inactivating the serine DD-transpeptidase catalytic site. Penicillin is a cyclic analogue of the D-Ala-D-Ala terminated carbonyl donors, therefore in the presence of this antibiotic, the reaction stops at the level of the serine ester-linked penicilloyl enzyme. Thus β-lactam antibiotics force these enzymes to behave like penicillin binding proteins.
Kinetically, the interaction between the DD-peptidase and β-lactams is a three-step reaction:
β-Lactams may form an adduct E-I* of high stability with . The half life of this adduct is around hours, whereas the half-life of the normal reaction is in the order of milliseconds.
The interference with the enzyme processes responsible for cell wall formation results in cellular lysis and death due to the triggering of the autolytic system in the bacteria.
See also
*Vancomycin
Vancomycin is a glycopeptide antibiotic medication used to treat certain bacterial infections. It is administered intravenously ( injection into a vein) to treat complicated skin infections, bloodstream infections, endocarditis, bone an ...
, an antibiotic that binds the D-ala-D-ala residues, inhibiting elongation via glycosyltransferase
References
External links
* The MEROPS online database for peptidases and their inhibitorsS11.001
* * {{Portal bar, Biology, border=no EC 3.4.16 Bacteria Microbial metabolism