Cyclosilicate on:
[Wikipedia]
[Google]
[Amazon]
 Silicate minerals are rock-forming
Silicate minerals are rock-forming  Living organisms also contribute to this geologic cycle. For example, a type of
Living organisms also contribute to this geologic cycle. For example, a type of
 Nesosilicates (from Greek 'island'), or orthosilicates, have the orthosilicate ion, present as isolated (insular)
Nesosilicates (from Greek 'island'), or orthosilicates, have the orthosilicate ion, present as isolated (insular)
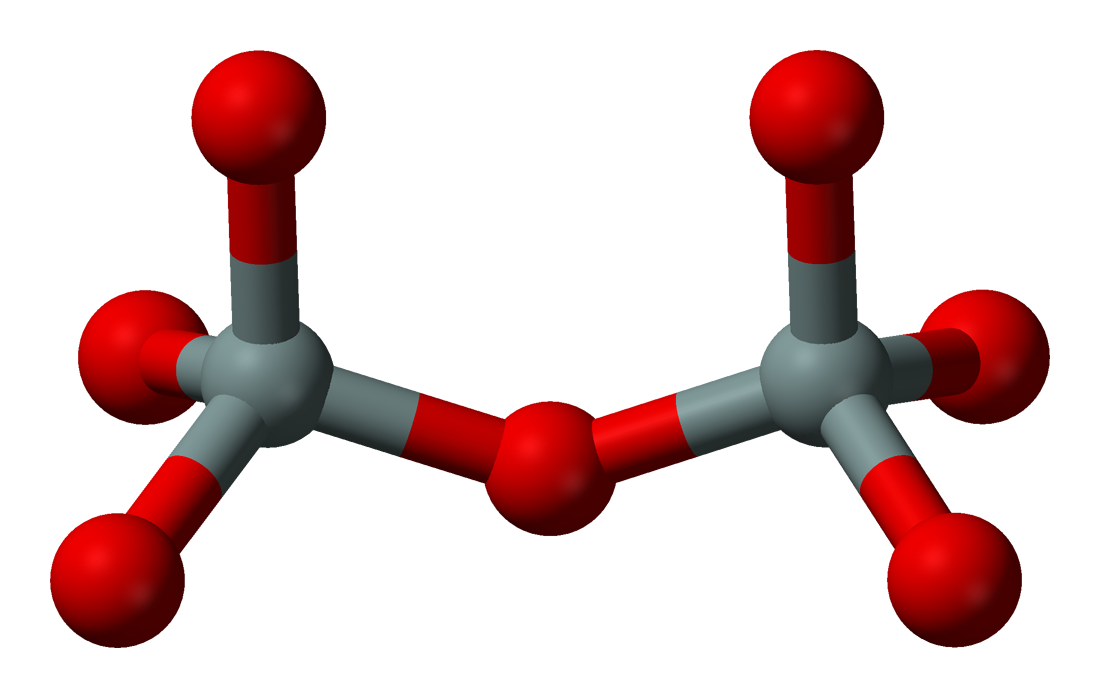 Sorosilicates (from Greek 'heap, mound') have isolated pyrosilicate anions , consisting of double tetrahedra with a shared oxygen vertex—a silicon:oxygen ratio of 2:7. The Nickel–Strunz classification is 09.B. Examples include:
* Thortveitite –
*
Sorosilicates (from Greek 'heap, mound') have isolated pyrosilicate anions , consisting of double tetrahedra with a shared oxygen vertex—a silicon:oxygen ratio of 2:7. The Nickel–Strunz classification is 09.B. Examples include:
* Thortveitite –
*
File:Beryll.ring.combined.png, 6 units ,
Some example minerals are:
* 3-member single ring
** Benitoite –
* 4-member single ring
** Papagoite – .
* 6-member single ring
**
File:Pyroxen-chain.png, Inosilicate, pyroxene family, with 2-periodic single chain , diopside
File:Tremolite-chain.png, Inosilicate, clinoamphibole, with 2-periodic double chains ,
File:Muskovite.sheet.png, Phyllosilicate, mica group,

 Tectosilicates, or "framework silicates," have a three-dimensional framework of silicate
Tectosilicates, or "framework silicates," have a three-dimensional framework of silicate
 Silicate minerals are rock-forming
Silicate minerals are rock-forming mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid substance with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. (2011): Mi ...
s made up of silicate
A silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is also used ...
groups. They are the largest and most important class of minerals and make up approximately 90 percent of Earth's crust
Earth's crust is its thick outer shell of rock, referring to less than one percent of the planet's radius and volume. It is the top component of the lithosphere, a solidified division of Earth's layers that includes the crust and the upper ...
.
In mineralogy
Mineralogy is a subject of geology specializing in the scientific study of the chemistry, crystal structure, and physical (including optical mineralogy, optical) properties of minerals and mineralized artifact (archaeology), artifacts. Specific s ...
, the crystalline forms of silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant f ...
(silicon dioxide, ) are usually considered to be tectosilicates
Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of Earth's crust.
In mineralogy, the crystalline forms of silica (silicon dio ...
, and they are classified as such in the Dana system (75.1). However, the Nickel-Strunz system classifies them as oxide mineral
The oxide mineral class includes those minerals in which the oxide anion (O2−) is bonded to one or more metal alloys. The hydroxide-bearing minerals are typically included in the oxide class. Minerals with complex anion groups such as the sil ...
s (4.DA). Silica is found in nature as the mineral quartz
Quartz is a hard, crystalline mineral composed of silica (silicon dioxide). The Atom, atoms are linked in a continuous framework of SiO4 silicon–oxygen Tetrahedral molecular geometry, tetrahedra, with each oxygen being shared between two tet ...
, and its polymorphs.
On Earth, a wide variety of silicate minerals occur in an even wider range of combinations as a result of the processes that have been forming and re-working the crust for billions of years. These processes include partial melting
Melting, or fusion, is a physical process that results in the phase transition of a substance from a solid to a liquid. This occurs when the internal energy of the solid increases, typically by the application of heat or pressure, which inc ...
, crystallization
Crystallization is a process that leads to solids with highly organized Atom, atoms or Molecule, molecules, i.e. a crystal. The ordered nature of a crystalline solid can be contrasted with amorphous solids in which atoms or molecules lack regu ...
, fractionation
Fractionation is a separation process in which a certain quantity of a mixture (of gasses, solids, liquids, enzymes, or isotopes, or a suspension) is divided during a phase transition, into a number of smaller quantities (fractions) in which t ...
, metamorphism
Metamorphism is the transformation of existing Rock (geology), rock (the protolith) to rock with a different mineral composition or Texture (geology), texture. Metamorphism takes place at temperatures in excess of , and often also at elevated ...
, weathering
Weathering is the deterioration of rocks, soils and minerals (as well as wood and artificial materials) through contact with water, atmospheric gases, sunlight, and biological organisms. It occurs '' in situ'' (on-site, with little or no move ...
, and diagenesis
Diagenesis () is the process of physical and chemical changes in sediments first caused by water-rock interactions, microbial activity, and compaction after their deposition. Increased pressure and temperature only start to play a role as sedi ...
.
 Living organisms also contribute to this geologic cycle. For example, a type of
Living organisms also contribute to this geologic cycle. For example, a type of plankton
Plankton are the diverse collection of organisms that drift in Hydrosphere, water (or atmosphere, air) but are unable to actively propel themselves against ocean current, currents (or wind). The individual organisms constituting plankton are ca ...
known as diatom
A diatom (Neo-Latin ''diatoma'') is any member of a large group comprising several Genus, genera of algae, specifically microalgae, found in the oceans, waterways and soils of the world. Living diatoms make up a significant portion of Earth's B ...
s construct their exoskeleton
An exoskeleton () . is a skeleton that is on the exterior of an animal in the form of hardened integument, which both supports the body's shape and protects the internal organs, in contrast to an internal endoskeleton (e.g. human skeleton, that ...
s ("frustules") from silica extracted from seawater
Seawater, or sea water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approximat ...
. The frustules of dead diatoms are a major constituent of deep ocean
The deep sea is broadly defined as the ocean depth where light begins to fade, at an approximate depth of or the point of transition from continental shelves to continental slopes. Conditions within the deep sea are a combination of low tempe ...
sediment
Sediment is a solid material that is transported to a new location where it is deposited. It occurs naturally and, through the processes of weathering and erosion, is broken down and subsequently sediment transport, transported by the action of ...
, and of diatomaceous earth
Diatomaceous earth ( ), also known as diatomite ( ), celite, or kieselguhr, is a naturally occurring, soft, siliceous rock, siliceous sedimentary rock that can be crumbled into a fine white to off-white powder. It has a particle size ranging fr ...
.
General structure
A silicate mineral is generally aninorganic compound
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemistry''.
Inorgan ...
consisting of subunits with the formula iO2+''n''sup>2''n''−. Although depicted as such, the description of silicates as anions is a simplification. Balancing the charges of the silicate anions are metal cations, M''x''+. Typical cations are Mg2+, Fe2+, and Na+. The Si-O-M linkage between the silicates and the metals are strong, polar-covalent bonds. Silicate anions ( iO2+''n''sup>2''n''−) are invariably colorless, or when crushed to a fine powder, white. The colors of silicate minerals arise from the metal component, commonly iron.
In most silicate minerals, silicon is tetrahedral, being surrounded by four oxides. The coordination number
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central ion ...
of the oxides is variable except when it bridges two silicon centers, in which case the oxide has a coordination number of two.
Some silicon centers may be replaced by atoms of other elements, still bound to the four corner oxygen corners. If the substituted atom is not normally tetravalent, it usually contributes extra charge to the anion, which then requires extra cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
s. For example, in the mineral orthoclase
Orthoclase, or orthoclase feldspar ( endmember formula K Al Si3 O8), is an important tectosilicate mineral which forms igneous rock. The name is from the Ancient Greek for "straight fracture", because its two cleavage planes are at right angles ...
, the anion is a tridimensional network of tetrahedra in which all oxygen corners are shared. If all tetrahedra had silicon centers, the anion would be just neutral silica . Replacement of one in every four silicon atoms by an aluminum
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
atom results in the anion , whose charge is neutralized by the potassium
Potassium is a chemical element; it has Symbol (chemistry), symbol K (from Neo-Latin ) and atomic number19. It is a silvery white metal that is soft enough to easily cut with a knife. Potassium metal reacts rapidly with atmospheric oxygen to ...
cations .
Main groups
Inmineralogy
Mineralogy is a subject of geology specializing in the scientific study of the chemistry, crystal structure, and physical (including optical mineralogy, optical) properties of minerals and mineralized artifact (archaeology), artifacts. Specific s ...
, silicate minerals are classified into seven major groups according to the structure of their silicate anion:
Tectosilicates can only have additional cations if some of the silicon is replaced by an atom of lower valence such as aluminum. Al for Si substitution is common.
Nesosilicates or orthosilicates
 Nesosilicates (from Greek 'island'), or orthosilicates, have the orthosilicate ion, present as isolated (insular)
Nesosilicates (from Greek 'island'), or orthosilicates, have the orthosilicate ion, present as isolated (insular) tetrahedra
In geometry, a tetrahedron (: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular Face (geometry), faces, six straight Edge (geometry), edges, and four vertex (geometry), vertices. The tet ...
connected only by interstitial cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
s. The Nickel–Strunz classification is 09.A –examples include:
*Phenakite group
** Phenakite –
** Willemite –
*Olivine
The mineral olivine () is a magnesium iron Silicate minerals, silicate with the chemical formula . It is a type of Nesosilicates, nesosilicate or orthosilicate. The primary component of the Earth's upper mantle (Earth), upper mantle, it is a com ...
group
**Forsterite
Forsterite (Mg2SiO4; commonly abbreviated as Fo; also known as white olivine) is the magnesium-rich Endmember, end-member of the olivine solid solution series. It is Isomorphism (crystallography), isomorphous with the iron-rich end-member, fayalit ...
–
**Fayalite
Fayalite (, commonly abbreviated to Fa) is the iron-rich endmember, end-member of the olivine solid solution, solid-solution series. In common with all minerals in the olivine, olivine group, fayalite crystallizes in the orthorhombic system (spac ...
–
** Tephroite –
*Garnet
Garnets () are a group of silicate minerals that have been used since the Bronze Age as gemstones and abrasives.
Garnet minerals, while sharing similar physical and crystallographic properties, exhibit a wide range of chemical compositions, de ...
group
**Pyrope
The mineral pyrope is a member of the garnet group. Pyrope is the only member of the garnet family to always display red colouration in natural samples, and it is from this characteristic that it gets its name: from the Greek words for ''fire'' ...
–
**Almandine
Almandine (), also known as almandite, is a mineral belonging to the garnet group. The name is a corruption of alabandicus, which is the name applied by Pliny the Elder to a stone found or worked at Alabanda, a town in Caria in Asia Minor. Alma ...
–
**Spessartine
Spessartine is a nesosilicate, manganese aluminium garnet species, Mn2+3Al2(SiO4)3. Gemological Institute of America, ''GIA Gem Reference Guide'' 1995, This mineral is sometimes mistakenly referred to as ''spessartite''.
Spessartine's name is ...
–
**Grossular
Grossular is a calcium-aluminium species of the garnet group of minerals. It has the chemical formula of Ca3Al2(SiO4)3 but the calcium may, in part, be replaced by ferrous iron and the aluminium by ferric iron. The name grossular is derived from ...
–
**Andradite
Andradite is a Mineralogy, mineral species of the Garnet, garnet group. It is a Silicate minerals#Nesosilicates, nesosilicate, with chemical formula Ca3Fe2Si3O12.
Andradite includes three varieties:
* ''Colophonite'': a historical variety found ...
–
**Uvarovite
Uvarovite is a chromium-bearing garnet group species with the formula: Ca3 Cr2( Si O4)3. It was discovered in 1832 by Germain Henri Hess who named it after Count Sergei Uvarov (1765–1855), a Russian statesman and amateur mineral collector. I ...
–
** Hydrogrossular –
*Zircon group
**Zircon
Zircon () is a mineral belonging to the group of nesosilicates and is a source of the metal zirconium. Its chemical name is zirconium(IV) silicate, and its corresponding chemical formula is Zr SiO4. An empirical formula showing some of th ...
–
** Thorite –
** Hafnon –
* group
**Andalusite
Andalusite is an aluminium nesosilicate mineral with the chemical formula Al2SiO5. This mineral was called andalousite by Delamétherie, who thought it came from Andalusia, Spain. It soon became clear that it was a locality error, and that the sp ...
–
**Kyanite
Kyanite is a typically blue aluminosilicate mineral, found in aluminium-rich metamorphic pegmatites and sedimentary rock. It is the high pressure Polymorphism (materials science), polymorph of andalusite and sillimanite, and the presence of kyani ...
–
**Sillimanite
Sillimanite or fibrolite is an aluminosilicate mineral with the chemical formula Al2SiO5. Sillimanite is named after the American chemist Benjamin Silliman (1779–1864). It was first described in 1824 for an occurrence in Chester, Connecticut ...
–
** Dumortierite –
**Topaz
Topaz is a silicate mineral made of aluminium, aluminum and fluorine with the chemical formula aluminium, Alsilicon, Sioxygen, O(fluorine, F, hydroxide, OH). It is used as a gemstone in jewelry and other adornments. Common topaz in its natural ...
–
** Staurolite –
* Humite group –
**Norbergite
Norbergite is a Silicate minerals, nesosilicate mineral with formula Magnesium, Mg3(Silicon, SiOxygen, O4)(Fluorine, F,hydroxide, OH)2. It is a member of the humite group.
It was first described in 1926 for an occurrence in the Östanmoss iron mi ...
–
** Chondrodite –
** Humite –
** Clinohumite –
* Datolite –
* Titanite –
* Chloritoid –
*Mullite
Mullite or porcelainite is a rare silicate mineral formed during contact metamorphism of clay minerals. It can form two stoichiometric forms: 3 Al2 O32 SiO2 or 2Al2O3 SiO2. Unusually, mullite has no charge-balancing cations present. As a result ...
(aka Porcelainite) –
Sorosilicates
 Sorosilicates (from Greek 'heap, mound') have isolated pyrosilicate anions , consisting of double tetrahedra with a shared oxygen vertex—a silicon:oxygen ratio of 2:7. The Nickel–Strunz classification is 09.B. Examples include:
* Thortveitite –
*
Sorosilicates (from Greek 'heap, mound') have isolated pyrosilicate anions , consisting of double tetrahedra with a shared oxygen vertex—a silicon:oxygen ratio of 2:7. The Nickel–Strunz classification is 09.B. Examples include:
* Thortveitite –
*Hemimorphite
Hemimorphite is the chemical compound Zinc, Zn4(Pyrosilicate, Si2O7)(Hydroxide, OH)2Water of crystallization, ·H2O, a component of mineral Calamine (mineral), calamine. It is a silicate mineral which, together with smithsonite (ZnCO3), has bee ...
(calamine
Calamine, also known as calamine lotion, is a medication made from powdered calamine (mineral), calamine mineral that is used to treat mild itchiness. Conditions treated include sunburn, insect bites, Toxicodendron radicans, poison ivy, poiso ...
) –
* Lawsonite –
* Axinite –
* Ilvaite –
*Epidote group (has both and groups}
**Epidote
Epidote is a calcium aluminium iron sorosilicate mineral.
Description
Well developed crystals of epidote, Ca2Al2(Fe3+;Al)(SiO4)(Si2O7)O(OH), crystallizing in the monoclinic system, are of frequent occurrence: they are commonly prismatic in ha ...
–
** Zoisite –
*** Tanzanite –
** Clinozoisite –
** Allanite –
** Dollaseite-(Ce) –
*Vesuvianite
Vesuvianite, also known as idocrase, is a green, brown, yellow, or blue silicate mineral. Vesuvianite occurs as tetragonal crystals in skarn deposits and limestones that have been subjected to contact metamorphism. It was first discovered withi ...
( idocrase) –
Cyclosilicates
Cyclosilicates (from Greek 'circle'), or ring silicates, have three or more tetrahedra linked in a ring. The general formula is (Si''x''O3''x'')2''x''−, where one or more silicon atoms can be replaced by other 4-coordinated atom(s). The silicon:oxygen ratio is 1:3. Double rings have the formula (Si2''x''O5''x'')2''x''− or a 2:5 ratio. The Nickel–Strunz classification is 09.C. Possible ring sizes include:beryl
Beryl ( ) is a mineral composed of beryllium aluminium Silicate minerals#Cyclosilicates, silicate with the chemical formula Be3Al2(SiO3)6. Well-known varieties of beryl include emerald and Aquamarine (gem), aquamarine. Naturally occurring Hex ...
(red: Si, blue: O)
File:Benitoid.2200.png, 3 units , benitoite
File:Papagoite.2200.png, 4 units , papagoite
File:Eudialyte.2200.png, 9 units , eudialyte
Eudialyte, whose name derives from the Greek phrase , , meaning "well decomposable", is a somewhat rare, nine-member-ring cyclosilicate mineral, which forms in alkaline igneous rocks, such as nepheline syenites. Its name alludes to its ready so ...
File:Milarite.png, 12 units, double ring , milarite
Beryl
Beryl ( ) is a mineral composed of beryllium aluminium Silicate minerals#Cyclosilicates, silicate with the chemical formula Be3Al2(SiO3)6. Well-known varieties of beryl include emerald and Aquamarine (gem), aquamarine. Naturally occurring Hex ...
–
** Bazzite –
** Sugilite –
**Tourmaline
Tourmaline ( ) is a crystalline silicate mineral, silicate mineral group in which boron is chemical compound, compounded with chemical element, elements such as aluminium, iron, magnesium, sodium, lithium, or potassium. This gemstone comes in a ...
–
**Pezzottaite
Pezzottaite, marketed under the name raspberyl or raspberry beryl, is a mineral species first recognized by the International Mineralogical Association in September 2003. Pezzottaite is a caesium analogue of beryl, a silicate of caesium, beryl ...
–
** Osumilite –
**Cordierite
Cordierite (mineralogy) or iolite (gemology) is a magnesium iron aluminium cyclosilicate. Iron is almost always present, and a solid solution exists between Mg-rich cordierite and Fe-rich sekaninaite with a series formula: to . A high-tempera ...
–
** Sekaninaite –
* 9-member single ring
** Eudialyte
Eudialyte, whose name derives from the Greek phrase , , meaning "well decomposable", is a somewhat rare, nine-member-ring cyclosilicate mineral, which forms in alkaline igneous rocks, such as nepheline syenites. Its name alludes to its ready so ...
–
* 6-member double ring
** Milarite –
The ring in axinite contains two B and four Si tetrahedra and is highly distorted compared to the other 6-member ring cyclosilicates.
Inosilicates
Inosilicates (from Greek enitive: 'fibre'), or chain silicates, have interlocking chains ofsilicate
A silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is also used ...
tetrahedra
In geometry, a tetrahedron (: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular Face (geometry), faces, six straight Edge (geometry), edges, and four vertex (geometry), vertices. The tet ...
with either , 1:3 ratio, for single chains or , 4:11 ratio, for double chains. The Nickel–Strunz classification is 09.D – examples include:
Single chain inosilicates
*Pyroxene
The pyroxenes (commonly abbreviated Px) are a group of important rock-forming inosilicate minerals found in many igneous and metamorphic rocks. Pyroxenes have the general formula , where X represents ions of calcium (Ca), sodium (Na), iron ( ...
group
**Enstatite – orthoferrosilite series
*** Enstatite –
*** Ferrosilite –
** Pigeonite –
**Diopside – hedenbergite series
*** Diopside –
*** Hedenbergite –
***Augite
Augite, also known as Augurite, is a common rock-forming pyroxene mineral with formula . The crystals are monoclinic and prismatic. Augite has two prominent cleavages, meeting at angles near 90 degrees.
Characteristics
Augite is a solid soluti ...
–
**Sodium pyroxene series
***Jadeite
Jadeite is a pyroxene mineral with composition Na Al Si2 O6. It is hard (Mohs hardness of about 6.5 to 7.0), very tough, and dense, with a specific gravity of about 3.4. It is found in a wide range of colors, but is most often found in shades ...
–
*** Aegirine (or acmite) –
** Spodumene –
** Pyroxferroite -
*Pyroxenoid group
**Wollastonite
Wollastonite is a calcium Silicate minerals, inosilicate mineral (calcium, Casilicon, Sioxygen, O3) that may contain small amounts of iron, magnesium, and manganese substituting for calcium. It is usually white. It forms when impure limestone or D ...
–
** Rhodonite –
** Pectolite –
Double chain inosilicates
*Amphibole
Amphibole ( ) is a group of inosilicate minerals, forming prism or needlelike crystals, composed of double chain tetrahedra, linked at the vertices and generally containing ions of iron and/or magnesium in their structures. Its IMA symbol is ...
group
** Anthophyllite –
**Cummingtonite series
*** Cummingtonite –
*** Grunerite –
**Tremolite series
***Tremolite
Tremolite is a member of the amphibole group of silicate minerals with composition Ca2(Mg5.0-4.5Fe2+0.0-0.5)Si8O22(OH)2. Tremolite forms by metamorphism of sediments rich in dolomite and quartz, and occurs in two distinct forms, crystals and fib ...
–
***Actinolite
Actinolite is an amphibole silicate mineral with the chemical formula .
Etymology
The name ''actinolite'' is derived from the Greek word ''aktis'' (), meaning "beam" or "ray", because of the mineral's fibrous nature.
Mineralogy
Actinolite i ...
–
**Hornblende
Hornblende is a complex silicate minerals#Inosilicates, inosilicate series of minerals. It is not a recognized mineral in its own right, but the name is used as a general or field term, to refer to a dark amphibole. Hornblende minerals are common ...
–
**Sodium amphibole group
*** Glaucophane –
***Riebeckite
Riebeckite is a sodium-rich member of the amphibole group of silicate minerals, chemical formula Na2(Fe2+3Fe3+2)Si8O22(OH)2. It forms a solid solution series with magnesioriebeckite. It crystallizes in the monoclinic system, usually as long ...
(asbestos
Asbestos ( ) is a group of naturally occurring, Toxicity, toxic, carcinogenic and fibrous silicate minerals. There are six types, all of which are composed of long and thin fibrous Crystal habit, crystals, each fibre (particulate with length su ...
) –
***Arfvedsonite
Arfvedsonite () or ''soda hornblende'' (partiellement obsolète) is a sodium amphibole mineral with composition: aNa2] Fe2+)4Fe3+(OH)2, Si8O22]. It crystallizes in the monoclinic prismatic crystal system and typically occurs as greenish black t ...
–
tremolite
Tremolite is a member of the amphibole group of silicate minerals with composition Ca2(Mg5.0-4.5Fe2+0.0-0.5)Si8O22(OH)2. Tremolite forms by metamorphism of sediments rich in dolomite and quartz, and occurs in two distinct forms, crystals and fib ...
File:Wollastonite-chain.png, Inosilicate, unbranched 3-periodic single chain of wollastonite
Wollastonite is a calcium Silicate minerals, inosilicate mineral (calcium, Casilicon, Sioxygen, O3) that may contain small amounts of iron, magnesium, and manganese substituting for calcium. It is usually white. It forms when impure limestone or D ...
File:Rhodonite-chain.png, Inosilicate with 5-periodic single chain, rhodonite
File:Pellyite-chain.png, Inosilicate with cyclic branched 8-periodic chain, pellyite
Phyllosilicates
Phyllosilicates (from Greek 'leaf'), or sheet silicates, form parallel sheets of silicate tetrahedra with or a 2:5 ratio. The Nickel–Strunz classification is 09.E. All phyllosilicate minerals arehydrate
In chemistry, a hydrate is a substance that contains water or its constituent elements. The chemical state of the water varies widely between different classes of hydrates, some of which were so labeled before their chemical structure was understo ...
d, with either water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
or hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
groups attached.
Examples include:
* Serpentine subgroup
** Antigorite –
**Chrysotile
Chrysotile or white asbestos is the most commonly encountered form of asbestos, accounting for approximately 95% of the asbestos in the United StatesOccupational Safety and Health Administration, U.S. Department of Labor (2007)29 C.F.R.&nb ...
–
** Lizardite –
*Clay minerals
Clay minerals are hydrous aluminium phyllosilicates (e.g. kaolin, Al2 Si2 O5( OH)4), sometimes with variable amounts of iron, magnesium, alkali metals, alkaline earths, and other cations found on or near some planetary surfaces.
Clay mineral ...
group
**1:1 clay minerals (TO)
***Halloysite
Halloysite is an aluminosilicate clay mineral with the empirical formula Al2Si2O5(OH)4. Its main constituents are oxygen (55.78%), silicon (21.76%), aluminium (20.90%), and hydrogen (1.56%). It is a member of the kaolinite group. Halloysite typic ...
–
***Kaolinite
Kaolinite ( ; also called kaolin) is a clay mineral, with the chemical composition Al2 Si2 O5( OH)4. It is a layered silicate mineral, with one tetrahedral sheet of silica () linked through oxygen atoms to one octahedral sheet of alumina () ...
–
**2:1 clay minerals (TOT)
***Pyrophyllite
Pyrophyllite is a phyllosilicate mineral composed of aluminium silicate hydroxide: Al2Si4O10(OH)2. It occurs in two forms (habits): crystalline folia and compact masses; distinct crystals are not known.
The folia have a pronounced pearly luster ...
–
***Talc
Talc, or talcum, is a clay mineral composed of hydrated magnesium silicate, with the chemical formula . Talc in powdered form, often combined with corn starch, is used as baby powder. This mineral is used as a thickening agent and lubricant ...
–
***Illite
Illite, also called hydromica or hydromuscovite, is a group of closely related non-expanding clay minerals. Illite is a secondary mineral precipitate, and an example of a phyllosilicate, or layered alumino-silicate. Its structure is a 2:1 sandw ...
–
***Montmorillonite
Montmorillonite is a very soft phyllosilicate group of minerals that form when they precipitate from water solution as microscopic crystals, known as clay. It is named after Montmorillon in France. Montmorillonite, a member of the smectite grou ...
(smectite) –
***Chlorite
The chlorite ion, or chlorine dioxide anion, is the halite (oxyanion), halite with the chemical formula of . A chlorite (compound) is a compound that contains this group, with chlorine in the oxidation state of +3. Chlorites are also known as s ...
–
***Vermiculite
Vermiculite is a hydrous phyllosilicate mineral which undergoes significant expansion when heated. Exfoliation occurs when the mineral is heated sufficiently; commercial furnaces can routinely produce this effect. Vermiculite forms by the weathe ...
–
**Other clay minerals
***Sepiolite
Sepiolite, also known in English by the German name meerschaum ( , ; ; meaning " sea foam"), is a soft white clay mineral, often used to make tobacco pipes (known as meerschaum pipes). A complex magnesium silicate, a typical chemical formula ...
–
*** Palygorskite (or attapulgite) –
*Mica
Micas ( ) are a group of silicate minerals whose outstanding physical characteristic is that individual mica crystals can easily be split into fragile elastic plates. This characteristic is described as ''perfect basal cleavage''. Mica is co ...
group
**Brittle mica group
*** Anandite –
*** Bityite –
*** Clintonite –
*** Margarite –
**Dioctahedral mica group
***Celadonite subgroup
**** Celadonite –
**** Aluminoceladonite –
***Glauconite
Glauconite is an iron potassium phyllosilicate ( mica group) mineral of characteristic green color which is very friable and has very low weathering resistance.
It crystallizes with a monoclinic geometry. Its name is derived from the Greek ...
–
***Muscovite
Muscovite (also known as common mica, isinglass, or potash mica) is a hydrated phyllosilicate mineral of aluminium and potassium with formula KAl2(Al Si3 O10)( F,O H)2, or ( KF)2( Al2O3)3( SiO2)6( H2O). It has a highly perfect basal cleavage y ...
–
****Fuchsite
Fuchsite, also known as chrome mica, is a chromium (Cr)-rich variety of the mineral muscovite, belonging to the mica group of phyllosilicate minerals, with the chemical formula .
Trivalent chromium replaces one of the aluminium (Al) atoms in t ...
– (Cr replaces Al in muscovite)
****Illite
Illite, also called hydromica or hydromuscovite, is a group of closely related non-expanding clay minerals. Illite is a secondary mineral precipitate, and an example of a phyllosilicate, or layered alumino-silicate. Its structure is a 2:1 sandw ...
– (K-deficient muscovite)
**** Mariposite – (Cr-bearing muscovite)
**** Phengite – (Fe/Mg-bearing muscovite)
*** Paragonite –
***Roscoelite
Roscoelite is a green mineral from the mica group that contains vanadium.
The chemical formula is potassium, K(vanadium, V3+, aluminium, Al, magnesium, Mg)2aluminum, Alsilicon, Si3oxygen, O10(Ohydrogen, H)2.
Crystals of roscoelite take on the mono ...
–
**Trioctahedral mica group
*** Aspidolite –
***Biotite
Biotite is a common group of phyllosilicate minerals within the mica group, with the approximate chemical formula . It is primarily a solid-solution series between the iron- endmember annite, and the magnesium-endmember phlogopite; more al ...
subgroup –
**** Annite –
****Phlogopite
Phlogopite is a yellow, greenish, or reddish-brown member of the mica family of phyllosilicates. It is also known as magnesium mica.
Phlogopite is the magnesium endmember of the biotite solid solution series, with the chemical formula KMg3AlSi3 ...
–
***Hendricksite
Hendricksite is a member of the trioctahedral micas group. The mineral was named by Clifford Frondel and Jun Ito in honor of Sterling B. Hendricks, Sterling Brown Hendricks, who studied micas. It was approved in 1966 by the International Mineralog ...
–
***Lepidolite
Lepidolite is the common name for a lilac-gray or rose-colored series of minerals in the mica group. The mineralogical name for this series is the polylithionite-trilithionite series. Lepidolite has a chemical formula of . It is the most abundan ...
(polylithionite-trilithionite series) –
*** Zinnwaldite series –
muscovite
Muscovite (also known as common mica, isinglass, or potash mica) is a hydrated phyllosilicate mineral of aluminium and potassium with formula KAl2(Al Si3 O10)( F,O H)2, or ( KF)2( Al2O3)3( SiO2)6( H2O). It has a highly perfect basal cleavage y ...
(red: Si, blue: O)
File:Apophyllite.sheet.png, Phyllosilicate, single net of tetrahedra with 4-membered rings, apophyllite
The name apophyllite refers to a specific group of phyllosilicates, a class of minerals. Originally, the group name referred to a specific mineral, but was redefined in 1978 to stand for a class of minerals of similar chemical makeup that compris ...
-(KF)-apophyllite-(KOH) series
File:Pyrosmalite.sheet.png, Phyllosilicate, single tetrahedral nets of 6-membered rings, pyrosmalite-(Fe)-pyrosmalite-(Mn) series
File:Zeophyllite.sheet.png, Phyllosilicate, single tetrahedral nets of 6-membered rings, zeophyllite
File:Carletonite.sheet.png, Phyllosilicate, double nets with 4- and 6-membered rings, carletonite
Tectosilicates

 Tectosilicates, or "framework silicates," have a three-dimensional framework of silicate
Tectosilicates, or "framework silicates," have a three-dimensional framework of silicate tetrahedra
In geometry, a tetrahedron (: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular Face (geometry), faces, six straight Edge (geometry), edges, and four vertex (geometry), vertices. The tet ...
with in a 1:2 ratio. This group comprises nearly 75% of the crust of the Earth
Earth is the third planet from the Sun and the only astronomical object known to Planetary habitability, harbor life. This is enabled by Earth being an ocean world, the only one in the Solar System sustaining liquid surface water. Almost all ...
. Tectosilicates, with the exception of the quartz group, are aluminosilicate
Aluminosilicate refers to materials containing anionic Si-O-Al linkages. Commonly, the associate cations are sodium (Na+), potassium (K+) and protons (H+). Such materials occur as minerals, coal combustion products and as synthetic materials, of ...
s. The Nickel–Strunz classifications are 9.F (tectosilicates without zeolitic ), 9.G (tectosilicates with zeolitic ), and 4.DA (quartz/silica group). Below is a list of tectosilicate minerals and their chemical formulas, organized by groups and series:
*Quartz group (silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant f ...
)
**Quartz
Quartz is a hard, crystalline mineral composed of silica (silicon dioxide). The Atom, atoms are linked in a continuous framework of SiO4 silicon–oxygen Tetrahedral molecular geometry, tetrahedra, with each oxygen being shared between two tet ...
–
**Tridymite
Tridymite is a high-temperature polymorphism (materials science), polymorph of silica and usually occurs as minute tabular white or colorless pseudo-hexagonal crystals, or scales, in cavities in felsic volcanic rocks. Its chemical formula is sili ...
–
**Cristobalite
Cristobalite ( ) is a mineral polymorph of silica that is formed at very high temperatures. It has the same chemical formula as quartz, Si O2, but a distinct crystal structure. Both quartz and cristobalite are polymorphs with all the members o ...
–
**Coesite
Coesite () is a form (polymorphism (materials science), polymorph) of silicon dioxide (silicon, Sioxide, O2) that is formed when very high pressure (2–3 gigapascals), and moderately high temperature (), are applied to quartz. Coesite was first ...
–
**Stishovite
Stishovite is an extremely hard, dense tetragonal form ( polymorph) of silicon dioxide. It is very rare on the Earth's surface; however, it may be a predominant form of silicon dioxide in the Earth, especially in the lower mantle.
Stishovite w ...
–
**Moganite
Moganite is a tectosilicate mineral with the chemical formula Si O2 (silicon dioxide) that was discovered in 1976. It was initially described as a new form of silica from specimens found in the Barranco de Medio Almud, in the municipality of M ...
–
**Chalcedony
Chalcedony ( or ) is a cryptocrystalline form of silica, composed of very fine intergrowths of quartz and moganite. These are both silica minerals, but they differ in that quartz has a trigonal crystal structure, while moganite is monoclinic ...
–
*Feldspar
Feldspar ( ; sometimes spelled felspar) is a group of rock-forming aluminium tectosilicate minerals, also containing other cations such as sodium, calcium, potassium, or barium. The most common members of the feldspar group are the ''plagiocl ...
group
**Alkali feldspar series (potassium feldspars or K-spar)
***Microcline
Microcline (KAlSi3O8) is an important igneous rock-forming tectosilicate mineral. It is a potassium-rich alkali feldspar. Microcline typically contains minor amounts of sodium. It is common in granite and pegmatites. Microcline forms during s ...
–
****Amazonite
Amazonite, also known as amazonstone, is a green tectosilicate mineral, a variety of the potassium feldspar called microcline. Its chemical formula is KAlSi3O8, which is Polymorphism (materials science), polymorphic to orthoclase.
Its name is ta ...
– green variety of microcline
***Orthoclase
Orthoclase, or orthoclase feldspar ( endmember formula K Al Si3 O8), is an important tectosilicate mineral which forms igneous rock. The name is from the Ancient Greek for "straight fracture", because its two cleavage planes are at right angles ...
–
**** Moonstone – opalescent variety of orthoclase
*** Anorthoclase –
***Sanidine
Sanidine is the high temperature form of potassium feldspar with a general formula K(AlSi3O8). Sanidine is found most typically in felsic volcanic rocks such as obsidian, rhyolite and trachyte. Sanidine crystallizes in the monoclinic crystal sys ...
–
**Plagioclase
Plagioclase ( ) is a series of Silicate minerals#Tectosilicates, tectosilicate (framework silicate) minerals within the feldspar group. Rather than referring to a particular mineral with a specific chemical composition, plagioclase is a continu ...
feldspar series
***Albite
Albite is a plagioclase feldspar mineral. It is the sodium endmember of the plagioclase solid solution series. It represents a plagioclase with less than 10% anorthite content. The pure albite endmember has the formula . It is a tectosilicat ...
(sodium endmember) –
***Oligoclase
Oligoclase is a rock-forming mineral belonging to the plagioclase feldspars. In chemical composition and in its crystallographic and physical characters it is intermediate between albite ( Na Al Si3 O8) and anorthite ( CaAl2Si2O8). The albite:ano ...
– (Na:Ca 90:10 to 70:30)
*** Andesine – (Na:Ca 50:50 to 70:30)
***Labradorite
Labradorite (( Ca, Na)( Al, Si)4 O8) is a calcium-enriched feldspar mineral first identified in Labrador, Canada, which can display an iridescent effect ( schiller).
Labradorite is an intermediate to calcic member of the plagioclase series. It ...
– (Na:Ca 30:70 to 50:50)
***Bytownite
Bytownite is a calcium rich member of the plagioclase solid solution series of feldspar minerals with composition between anorthite and labradorite. It is usually defined as having between 70 and 90% An (formula: ). Like others of the series, byt ...
– (Na:Ca 10:90 to 30:70)
***Anorthite
Anorthite (< ''an'' 'not' + ''ortho'' 'straight') is the
Buddingtonite —
***
Mindat.org, Dana classification
Webmineral : Dana's New Silicate Classification
{{Authority control pl:Krzemiany
Celsian
Celsian is an uncommon feldspar mineral, barium aluminosilicate, Barium, BaAluminium, Al2Silicon, Si2Oxygen, O8. The mineral occurs in contact metamorphic rocks with significant barium content. Its crystal system is monoclinic, and it is white, ...
–
***Hyalophane
Hyalophane or jaloallofane is a crystalline mineral, part of the feldspar group of tectosilicates. It is considered a barium-rich potassium feldspar. Its chemical formula is , and it has a hardness of 6 to . The name ''hyalophane'' comes from the ...
–
*** Rubicline –
*Feldspathoid
The feldspathoids are a group of tectosilicate minerals which resemble feldspar
Feldspar ( ; sometimes spelled felspar) is a group of rock-forming aluminium tectosilicate minerals, also containing other cations such as sodium, calcium, pota ...
group
**Cancrinite subgroup
*** Cancrinite –
*** Afghanite –
*** Alloriite –
*** Bystrite –
*** Farneseite –
*** Sacrofanite –
*** Vishnevite –
** Danalite –
** Kalsilite –
**Leucite
Leucite (from the Greek word ''leukos'' meaning white) is a rock-forming mineral of the feldspathoid group, silica-undersaturated and composed of potassium and aluminium tectosilicate KAlSi2O6. Crystals have the form of cubic icositetrahedra b ...
–
**Nepheline subgroup
***Nepheline
Nepheline, also called nephelite (), is a rock-forming mineral in the feldspathoid groupa silica-undersaturated aluminosilicate, Na3 K Al4 Si4 O16, that occurs in intrusive and volcanic rocks with low silica, and in their associated pegmatit ...
–
*** Davidsmithite –
**Sodalite subgroup
***Sodalite
Sodalite ( ) is a tectosilicate mineral with the formula , with royal blue varieties widely used as an ornamental gemstone. Although massive sodalite samples are opaque, crystals are usually transparent to translucent. Sodalite is a member of th ...
–
***Hauyne
Hauyne or haüyne, also called hauynite or haüynite ( ), old name ''Azure spar'',The Edinburgh Encyclopaedia, 1st American ed., Volume 13. — J. and E. Parker edition, 1832. is a rare tectosilicate sulfate mineral with endmember formula . As ...
–
*** Lazurite –
*** Nosean –
***Tugtupite
Tugtupite is a beryllium aluminium tectosilicate. It also contains sodium and chlorine and has the formula Na4 Al Be Si4 O12 Cl. Tugtupite is a member of the silica-deficient feldspathoid mineral group. It occurs in high alkali intrusive igneou ...
–
* Scapolite group
** Marialite –
** Meionite –
*Zeolite
Zeolites are a group of several microporous, crystalline aluminosilicate minerals commonly used as commercial adsorbents and catalysts. They mainly consist of silicon, aluminium, oxygen, and have the general formula ・y where is either a meta ...
group
** Amicite –
**Analcime
Analcime (; ) or analcite is a white, gray, or colorless tectosilicate mineral. Analcime consists of hydrated sodium aluminium silicate in cubic crystalline form. Its chemical formula is NaAlSi2O6 · H2O. Minor amounts of potassium and calcium ...
–
**Brewsterite
Brewsterite is the name of a series of tectosilicate minerals of the zeolite group. Prior to 1997, brewsterite was recognized as a mineral species, but a reclassification in 1997 by the International Mineralogical Association changed it to a se ...
subgroup –
**Chabazite-Lévyne subgroup
*** Chabazite –
*** Lévyne –
** Clinoptilolite subgroup –
**Cowlesite
Cowlesite is a mineral named after American mineralogist John Cowles. W.S. Wise and Rudy W. Tschernich first described it in material from roadcuts along Neer Road, Goble, Oregon, United States. The description also incorporated data from S ...
–
** Dachiardite-K –
**Edingtonite
Edingtonite is a white, gray, brown, colorless, pink or yellow zeolite mineral. Its chemical formula is Ba Al2 Si3 O10·4 H2O. It has varieties with tetragonal, orthorhombic or triclinic crystals.
The mineral occurs within cavities in nepheline ...
–
**Erionite
Erionite is a naturally occurring fibrous mineral that belongs to a group of minerals called zeolites. It usually is found in volcanic ash that has been altered by weathering and ground water. Erionite forms brittle, wool-like fibrous masses i ...
subgroup –
**Faujasite
Faujasite (FAU-type zeolite) is a mineral group in the zeolite family of silicate minerals. The group consists of faujasite-Na, faujasite-Mg and faujasite-Ca. They all share the same basic formula by varying the amounts of sodium, magnesium and ...
subgroup –
** Ferrierite subgroup – (Ferrierite-Mg)
** Garronite-Ca –
** Gismondine – (Gismondine-Ca)
** Gmelinite subgroup – (Gmelinite-Na)
**Heulandite
Heulandite is the name of a series of tectosilicate minerals of the zeolite group. Prior to 1997, heulandite was recognized as a mineral species, but a reclassification in 1997 by the International Mineralogical Association changed it to a seri ...
subgroup –
** Hsianghualite –
** Laumontite –
** Mordenite –
** Nabesite –
**Natrolite subgroup
*** Natrolite –
*** Gonnardite –
*** Mesolite –
*** Scolecite –
** Paulingite subgroup – (Paulingite-K)
**Phillipsite subgroup
*** Phillipsite – (Phillipsite-Ca)
***Harmotome
Harmotome is a mineral, one of the rarer zeolites; a hydrated barium silicate with formula: . It forms vitreous white well defined monoclinic crystals, often associated with calcite and other zeolites. It has a Mohs hardness of 4 to 5 and a speci ...
–
**Pollucite
Pollucite is a zeolite mineral with the formula with iron, calcium, rubidium and potassium as common substituting elements. It is important as a significant ore of caesium and sometimes rubidium. It forms a solid solution series with analcime. I ...
–
**Stilbite subgroup
***Stilbite
Stilbite is the name of a series of tectosilicate minerals of the zeolite group. Prior to 1997, stilbite was recognized as a mineral species, but a reclassification in 1997 by the International Mineralogical Association changed it to a series nam ...
–
*** Barrerite –
*** Stellerite –
** Thomsonite subgroup – (Thomsonite-Ca)
**Wairakite
Wairakite is a zeolite mineral with an analcime structure but containing a calcium ion. The chemical composition is Ca8(Al16Si32O96)•16H2O. It is named for the location of its discovery in Wairakei, North Island, New Zealand, by Czechoslovakian ...
–
** Yugawaralite –
See also
* * *References
External links
Mindat.org, Dana classification
Webmineral : Dana's New Silicate Classification
{{Authority control pl:Krzemiany