Cyanation on:
[Wikipedia]
[Google]
[Amazon]
In organic synthesis, cyanation is the attachment or substitution of a
 A variety of mechanistically distinct pathways are known to cyanate arenes:
A variety of mechanistically distinct pathways are known to cyanate arenes:
 In addition, palladium-catalyzed cyanations of aryl halides have been extensively explored. Generally, KCN or its less toxic surrogate Zn(CN)2 are used as nucleophilic cyanide sources. To further diminish toxicity concerns, potassium ferricyanide has also been used as a cyanide source. Catalytic cycles are believed to proceed through a standard Pd (0/II) pathway with reductive elimination forging the key C-C bond. Deactivation of Pd(II) with excess cyanide is a common problem. Palladium catalysis conditions for aryl iodides, bromides, and even chlorides have been developed:
In addition, palladium-catalyzed cyanations of aryl halides have been extensively explored. Generally, KCN or its less toxic surrogate Zn(CN)2 are used as nucleophilic cyanide sources. To further diminish toxicity concerns, potassium ferricyanide has also been used as a cyanide source. Catalytic cycles are believed to proceed through a standard Pd (0/II) pathway with reductive elimination forging the key C-C bond. Deactivation of Pd(II) with excess cyanide is a common problem. Palladium catalysis conditions for aryl iodides, bromides, and even chlorides have been developed:
 Nickel-catalyzed cyanations avoid the use of precious metals, and can take advantage of benzyl cyanide or
Nickel-catalyzed cyanations avoid the use of precious metals, and can take advantage of benzyl cyanide or 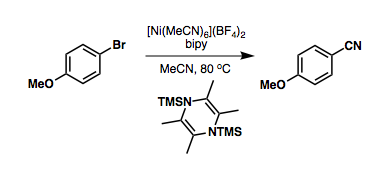 Sandmeyer cyanation is a means of converting aniline derivatives to benzonitriles. The cyanation is generally postulated to be two-electron, while with
Sandmeyer cyanation is a means of converting aniline derivatives to benzonitriles. The cyanation is generally postulated to be two-electron, while with


cyanide
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms.
In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of a ...
group on various substrate
Substrate may refer to:
Physical layers
*Substrate (biology), the natural environment in which an organism lives, or the surface or medium on which an organism grows or is attached
** Substrate (locomotion), the surface over which an organism lo ...
s. Such transformations are high-value because they generate C-C bond. Furthermore nitrile
In organic chemistry, a nitrile is any organic compound that has a functional group. The prefix ''cyano-'' is used interchangeably with the term ''nitrile'' in industrial literature. Nitriles are found in many useful compounds, including met ...
s are versatile functional groups.
Cyanation to form sp3 nitriles
Typically, alkyl nitriles are formed ''via'' SN1 or SN2-type cyanation with alkyl electrophiles. Illustrative is the synthesis of benzyl cyanide by the reaction ofbenzyl chloride
Benzyl chloride, or α-chlorotoluene, is an organic compound with the formula C6H5CH2Cl. This colorless liquid is a reactive organochlorine compound that is a widely used chemical building block.
Preparation
Benzyl chloride is prepared indust ...
and sodium cyanide. In some cases cuprous cyanide is used instead of sodium cyanide.
Cyanation of ketones or aldehydes yields the corresponding cyanohydrins
In organic chemistry, a cyanohydrin or hydroxynitrile is a functional group found in organic compounds in which a Cyanide, cyano and a hydroxy group are attached to the same carbon atom. The general formula is , where R is H, alkyl, or aryl. Cy ...
, which can be done directly with the cyanide ion (the cyanohydrin reaction) or by using bisulfite, followed by displacement of sulfite:
A related reaction is hydrocyanation, which installs the elements of H-CN.
Cyanation of arenes
Cyanation of arenes offers access to benzoic acid derivatives, as well as the utility of aryl nitriles themselves in as fine chemicals: A variety of mechanistically distinct pathways are known to cyanate arenes:
A variety of mechanistically distinct pathways are known to cyanate arenes:
With arene as two-electron electrophile
While the classical Rosenmund Von-Braun reaction utilizes stoichiometric copper(I) cyanide as a cyanation source, newer variants have been developed that are catalytic in copper: In addition, palladium-catalyzed cyanations of aryl halides have been extensively explored. Generally, KCN or its less toxic surrogate Zn(CN)2 are used as nucleophilic cyanide sources. To further diminish toxicity concerns, potassium ferricyanide has also been used as a cyanide source. Catalytic cycles are believed to proceed through a standard Pd (0/II) pathway with reductive elimination forging the key C-C bond. Deactivation of Pd(II) with excess cyanide is a common problem. Palladium catalysis conditions for aryl iodides, bromides, and even chlorides have been developed:
In addition, palladium-catalyzed cyanations of aryl halides have been extensively explored. Generally, KCN or its less toxic surrogate Zn(CN)2 are used as nucleophilic cyanide sources. To further diminish toxicity concerns, potassium ferricyanide has also been used as a cyanide source. Catalytic cycles are believed to proceed through a standard Pd (0/II) pathway with reductive elimination forging the key C-C bond. Deactivation of Pd(II) with excess cyanide is a common problem. Palladium catalysis conditions for aryl iodides, bromides, and even chlorides have been developed:
 Nickel-catalyzed cyanations avoid the use of precious metals, and can take advantage of benzyl cyanide or
Nickel-catalyzed cyanations avoid the use of precious metals, and can take advantage of benzyl cyanide or acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not clas ...
as a cyanide source, ''via'' reductive C-C bond cleavage:
 Sandmeyer cyanation is a means of converting aniline derivatives to benzonitriles. The cyanation is generally postulated to be two-electron, while with
Sandmeyer cyanation is a means of converting aniline derivatives to benzonitriles. The cyanation is generally postulated to be two-electron, while with radical
Radical may refer to:
Politics and ideology Politics
*Radical politics, the political intent of fundamental societal change
*Radicalism (historical), the Radical Movement that began in late 18th century Britain and spread to continental Europe and ...
mediators in absence of metals, the reaction is likely radical.
With arene as a two-electron nucleophile
Metalated arenes can be cyanated withelectrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carri ...
cyanide sources, including cyanamides, cyanates, dimethylmalononitrile, or ethyl (ethoxymethylene)cyanoacetate. These methods can proceed with or without transition metal mediation:

With arene as a radical electrophile
Radical approaches to arene C-H cyanation are known. Photoredox mediators (metallic or organic) are most common:
References
{{Reflist Chemical reactions