Covalent Adaptable Network on:
[Wikipedia]
[Google]
[Amazon]
Covalent adaptable networks (CANs) are a type of
 Polyurethane (PU)
Polyurethane (PU)
 In recent years,
In recent years,
polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
material that closely resemble thermosetting polymer
In materials science, a thermosetting polymer, often called a thermoset, is a polymer that is obtained by irreversibly hardening (" curing") a soft solid or viscous liquid prepolymer (resin). Curing is induced by heat or suitable radiation and ...
s (thermosets). However, they are distinguished from thermosets by the incorporation of dynamic covalent chemistry into the polymer network. When a stimulus (for example heat, light, pH, ...) is applied to the material, these dynamic bonds become active and can be broken or exchanged with other pending functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
s, allowing the polymer network to change its topology. This introduces reshaping, (re)processing and recycling
Recycling is the process of converting waste materials into new materials and objects. This concept often includes the recovery of energy from waste materials. The recyclability of a material depends on its ability to reacquire the propert ...
into thermoset-like materials.
Background
Historically, polymer materials have always been subdivided in two categories based on their thermomechanical behaviour.Thermoplastic
A thermoplastic, or thermosoftening plastic, is any plastic polymer material that becomes pliable or moldable at a certain elevated temperature and solidifies upon cooling.
Most thermoplastics have a high molecular weight. The polymer chains as ...
polymer materials melt upon heating and become viscous liquids, whereas thermosetting polymer materials remain solid as a result of cross-link
In chemistry and biology, a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural ...
ing.
Thermoplastics consist of long polymer chains that are stiff at service temperatures but become softer with increasing temperature. At low temperatures, the molecular motion of the polymer chains is limited due to chain-entanglements, resulting in a hard and glassy material. Increasing the temperature will lead to a transition from a hard to a soft material at the glass transition temperature
The glass–liquid transition, or glass transition, is the gradual and reversible transition in amorphous materials (or in amorphous regions within semicrystalline materials) from a hard and relatively brittle "glassy" state into a viscous or rub ...
(Tg) yielding a visco-elastic liquid. In the case of (semi-)crystalline polymer materials, viscous flow is achieved when the melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state of matter, state from solid to liquid. At the melting point the solid and liquid phase (matter), phase exist in Thermodynamic equilib ...
(Tm) is reached and the intermolecular force
An intermolecular force (IMF; also secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles (e.g. ...
s in the ordered crystalline domain are overcome. Thermoplastics regain their solid properties upon cooling and can thus be reshaped by polymer processing methods such as extrusion
Extrusion is a process used to create objects of a fixed cross section (geometry), cross-sectional profile by pushing material through a Die (manufacturing), die of the desired cross-section. Its two main advantages over other manufacturing pro ...
and injection moulding
Injection moulding (U.S. spelling: injection molding) is a manufacturing process for producing parts by injecting molten material into a mould, or mold. Injection moulding can be performed with a host of materials mainly including metals (for ...
and they can also be recycled. Examples of thermoplastic polymers are polystyrene
Polystyrene (PS) is a synthetic polymer made from monomers of the aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It i ...
, polycarbonate
Polycarbonates (PC) are a group of thermoplastic polymers containing carbonate ester, carbonate groups in their chemical structures. Polycarbonates used in engineering are strong, toughness, tough materials, and some grades are optically transp ...
, polyethylene
Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging (plastic bags, plastic films, geomembranes and containers including bott ...
, nylon
Nylon is a family of synthetic polymers characterised by amide linkages, typically connecting aliphatic or Polyamide#Classification, semi-aromatic groups.
Nylons are generally brownish in color and can possess a soft texture, with some varieti ...
, Acrylonitrile butadiene styrene
Acrylonitrile butadiene styrene (ABS) (chemical formula (C8H8)''x''·(C4H6)''y''·(C3H3N)''z'' ) is a common thermoplastic polymer. Its glass transition temperature is approximately . ABS is amorphous and therefore has no true melting point.
A ...
(ABS), etc.
Thermosets, on the other hand, are three-dimensional networks that are formed through permanent chemical cross-linking of multifunctional compounds. This is an irreversible process that results in infusible and insoluble polymer networks with superior properties compared to most thermoplastics. When a thermoset is exposed to heat, it maintains its dimensional stability and thus cannot be reshaped. These polymer materials are generally used for demanding applications (''e.g.'' wind turbine
A wind turbine is a device that wind power, converts the kinetic energy of wind into electrical energy. , hundreds of thousands of list of most powerful wind turbines, large turbines, in installations known as wind farms, were generating over ...
s, aerospace
Aerospace is a term used to collectively refer to the atmosphere and outer space. Aerospace activity is very diverse, with a multitude of commercial, industrial, and military applications. Aerospace engineering consists of aeronautics and astron ...
, etc.) that require chemical resistance, dimensional stability and good mechanical properties. Typical thermosetting materials include epoxy resins
Epoxy is the family of basic components or Curing (chemistry), cured end products of epoxy Resin, resins. Epoxy resins, also known as polyepoxides, are a class of reactive prepolymers and polymers which contain epoxide groups. The epoxide fun ...
, polyester resin
Polyester resins are synthetic resins formed by the reaction of dibasic organic acids and polyhydric alcohols. Maleic anhydride is a commonly used raw material with diacid functionality in unsaturated polyester resins. Unsaturated polyester r ...
s, polyurethane
Polyurethane (; often abbreviated PUR and PU) is a class of polymers composed of organic chemistry, organic units joined by carbamate (urethane) links. In contrast to other common polymers such as polyethylene and polystyrene, polyurethane term ...
s, etc.
In the framework of sustainability, the combination of the mechanical properties of thermosets with the reprocessability of thermoplastics through the introduction of dynamic bonds has been the topic of numerous research studies. The use of non-covalent interactions such as hydrogen bond
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, Covalent bond, covalently b ...
ing, pi-stacking or crystallization that lead to physical cross-links between polymer chains is one way of introducing dynamic cross-linking. The thermoreversible nature of the physical cross-links results in polymer materials with improved mechanical properties without losing reprocessability. The properties of these physical networks are highly dependent on the used backbone and type of non-covalent interactions, but typically they are brittle
A material is brittle if, when subjected to stress, it fractures with little elastic deformation and without significant plastic deformation. Brittle materials absorb relatively little energy prior to fracture, even those of high strength. ...
at low temperature and become elastic or rubbery above Tg. Upon further heating, the physical cross-links disappear and the material behaves as a visco-elastic liquid, allowing it to be reprocessed. These materials are also known as thermoplastic elastomer
Thermoplastic elastomers (TPE), sometimes referred to as thermoplastic rubbers (TPR), are a class of copolymers or a physical mix of polymers (usually a plastic and a rubber) that consist of materials with both thermoplastic and elastomeric prop ...
s.
Covalent adaptable networks (CANs) instead use dynamic covalent bonds that are able to undergo exchange reactions upon application of an external stimulus, typically heat or light. In absence of a stimulus, these materials behave as thermosets, showing high chemical resistance and dimensional stability, but when the stimulus is applied, the dynamic bonds become activated, enabling the network to rearrange its topology on a molecular level. As a result, these materials are able to undergo permanent deformations, enabling reshaping, reprocessing, self-healing, etc. As such, CANs can be seen as an intermediate bridge between thermosets and thermoplastics.
In 2011, the research group of French researcher Ludwik Leibler developed a specific class of CANs based on an associative exchange mechanism (see subsection Classification). By adding a suitable catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
to epoxy/acid polyester based networks, they were able to prepare a permanent epoxy network that showed a gradual viscosity decrease upon heating. This type of behaviour is typical for vitreous silica and had never before been seen in organic polymer materials. Therefore, the authors introduced the name Vitrimers
Vitrimers are a class of plastics, which are derived from thermosetting polymers (thermosets) and are very similar to them. Vitrimers consist of molecular, covalent networks, which can change their topology by thermally activated bond-exchange rea ...
for these kind of materials.
Recent advancements in the field of CANs have shown their potential to someday replace conventional non-recyclable thermosetting materials. The exponential growth of publications involving CANs seen in literature indicate the increasing interest from academia. Additionally, there's also a growing interest in CANs from industry with, for example, the first vitrimer start-up company Mallinda and multiple European Union
The European Union (EU) is a supranational union, supranational political union, political and economic union of Member state of the European Union, member states that are Geography of the European Union, located primarily in Europe. The u ...
funded research projects with collaborations between academic and industry partners (such as Vitrimat, PUReSmart and NIPU-EJD).
Classification
CANs are currently subdivided in two groups, dissociative CANs and associative CANs, based on the underlying mechanism of the bond exchange reactions (''i.e.'' the order in which the bond forming and breaking occurs) and their resulting temperature dependence.Dissociative CANs
The exchange mechanism of dissociative CANs requires a bond-breaking event prior to the formation of a new bond (''i.e.'' an elimination/addition pathway). Upon application of a stimulus, theequilibrium
Equilibrium may refer to:
Film and television
* ''Equilibrium'' (film), a 2002 science fiction film
* '' The Story of Three Loves'', also known as ''Equilibrium'', a 1953 romantic anthology film
* "Equilibrium" (''seaQuest 2032'')
* ''Equilibr ...
shifts to the dissociated state, resulting in a temporarily decreased cross-link density in the network. When a sufficient amount of dynamic bonds dissociate due to the equilibrium being shifted below the gel point, the material will suffer a loss of dimensional stability and show a sudden and drastic viscosity
Viscosity is a measure of a fluid's rate-dependent drag (physics), resistance to a change in shape or to movement of its neighboring portions relative to one another. For liquids, it corresponds to the informal concept of ''thickness''; for e ...
decrease. After removal of the stimulus, the bonds reform and, in the ideal case, the original cross-link density is restored. This temporary decrease in cross-link density enables very fast topology rearrangements in dissociative CANs, such as viscous flow and stress relaxation
In materials science, stress relaxation is the observed decrease in stress in response to strain generated in the structure. This is primarily due to keeping the structure in a strained condition for some finite interval of time hence causing som ...
, which allows the reprocessing of covalently cross-linked polymer networks. Additionally, dissociative CANs can be solubilized in good solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
s.
Associative CANs
In contrast to dissociative CANs, networks in associative CANs do not depolymerize upon application of a stimulus and maintain a near constant cross-link density. Here, the exchange mechanism relies on the formation of a new bond before fragmentation of another bond (''i.e.'' an addition/elimination pathway). This means that bond exchange occurs via a temporarily more cross-linked intermediate state. However, in practice, this small increase will often be negligible, resulting in a practically constant cross-link density. As a result, associative CANs typically remain insoluble in inert solvents, even at elevated temperatures, although it has become apparent that some associative CANs can be dissolved in a good solvent. In the case of Vitrimers, associative exchange is triggered by heat and the viscosity of these materials is controlled by chemical exchange reactions, leading to a linear dependence of viscosity with inverse temperature according to the Arrhenius law. The decreased viscosity caused by rapid dynamic bond exchanges enables stress relaxation and network topology rearrangements in these materials.Applications
Recycling of PU foams
 Polyurethane (PU)
Polyurethane (PU) foam
Foams are two-phase materials science, material systems where a gas is dispersed in a second, non-gaseous material, specifically, in which gas cells are enclosed by a distinct liquid or solid material. Note, this source focuses only on liquid ...
s are highly versatile engineering materials used for a wide range of applications such as mattress
A mattress is a large, usually rectangular pad for supporting a person Lying (position), lying down, especially for sleeping. It is designed to be used as a bed, or on a bed frame as part of a bed. Mattresses may consist of a Quilting, quilted o ...
es, insulation, automotive, footwear and construction materials. Conventional PU foams are cross-linked materials or thermosets. PU foams can either be mechanically recycled (where PU foams are grinded and used as fillers
In animal feed, a filler is an ingredient added to provide dietary fiber, bulk or some other non-nutritive purpose. Products like corn fiber (corncobs), fruit fibers (pulp), rice bran, and whole grains are possible fillers.
Purpose
As source ...
), or chemically recycled (where PU foams are downcycled into polyols or other monomeric components via chemical degradation). However, most PU foams end up on landfill
A landfill is a site for the disposal of waste materials. It is the oldest and most common form of waste disposal, although the systematic burial of waste with daily, intermediate and final covers only began in the 1940s. In the past, waste was ...
s.
Currently, CANs are being investigated as a replacement for conventional foams, which would allow for easier recyclability of PU waste. For example, it was shown recently that the incorporation of disulfide
In chemistry, a disulfide (or disulphide in British English) is a compound containing a functional group or the anion. The linkage is also called an SS-bond or sometimes a disulfide bridge and usually derived from two thiol groups.
In inorg ...
bonds in PU foams led to their malleability and reprocessability into elastomer
An elastomer is a polymer with viscoelasticity (i.e. both viscosity and elasticity) and with weak intermolecular forces, generally low Young's modulus (E) and high failure strain compared with other materials. The term, a portmanteau of ''ela ...
s. Another possible solution is the addition of catalyst to post-consumer PU, which activates the exchange of urethane bonds and makes them reprocessable .
Self-healing materials
Polymer networks are susceptible to damage during their use. Self-healing is a promising tool to increase the lifetime and performance of the polymer, while simultaneously reducingplastic waste
Plastic pollution is the accumulation of plastic objects and particles (e.g. plastic bottles, bags and microbeads) in the Earth's environment that adversely affects humans, wildlife and their habitat. Plastics that act as pollutants are cate ...
.
Self-healing can operate via extrinsic or intrinsic mechanisms. Extrinsic systems rely on the incorporation of small capsules containing healing agents that get released during damage/cracking and heal the material, while intrinsic systems are inherently able to restore their integrity through, for example, incorporation of dynamic bonds into the polymer network. A known example of intrinsic self-healing is thermally healable crosslinked networks with Diels-Alder adducts, but various other chemistries have also been investigated, including transesterification
Transesterification is the process of exchanging the organic functional group R″ of an ester with the organic group R' of an alcohol. These reactions are often catalyzed by the addition of an acid or base catalyst. Strong acids catalyze the r ...
, olefin metathesis, and alkoxyamine chemistry. For polyimine
Polyimines are classified as polymer materials that contain imine groups, which are characterised by a double bond between a carbon and nitrogen atom. The term polyimine can also be found occasionally in covalent organic frameworks (COFs). In (ol ...
CANs, it has been shown that self-healing can already occur at room temperature. However, additional heating may still significantly reduce the amount of time required.
Another promising strategy involves light-activated systems, such as photothermal and photoreversible chemistry. For photothermal systems, the healing is triggered by heating, even if light is the transient stimulus that makes the healing possible. Dynamic exchange reactions are also often activated by direct infrared heating with the assistance of photothermal fillers (''e.g.'' carbon black
Carbon black (with subtypes acetylene black, channel black, furnace black, lamp black and thermal black) is a material produced by the incomplete combustion of coal tar, vegetable matter, or petroleum products, including fuel oil, fluid cataly ...
, graphene
Graphene () is a carbon allotrope consisting of a Single-layer materials, single layer of atoms arranged in a hexagonal lattice, honeycomb planar nanostructure. The name "graphene" is derived from "graphite" and the suffix -ene, indicating ...
, and gold nanoparticles). Self-healing materials based on direct photoreversible chemistry in principle don't involve heating. Some examples of this include the systems based on photoreversible cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more Unsaturated hydrocarbon, unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of th ...
that require ultraviolet (UV) irradiation, as well as photo-triggered radical reshufflings of sulfur-based dynamic covalent bonds.
Nanocomposites
Thermosets are currently in high demand for high-performance composites that are heavily needed in lightweight engineering and ultrahigh-performance mechanical parts. Applications include: packaging, remediation, energy storage, electromagnetic absorption, sensing and actuation, transportation and safety, defense systems, thermal flow control, information industry, catalysts, cosmetics, sports, etc. Such materials consist of a “soft” polymer phase that is combined withnanoparticle
A nanoparticle or ultrafine particle is a particle of matter 1 to 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 100 nm in only two directions. At ...
s dispersed in the polymer phase. The shape of these nanoparticles can vary wildly, from rods to spheres to platelets, to fibres, etc.
The unique thermo-responsive properties of CANs, induced by bond exchange kinetics, open interesting possibilities for the introduction of property switches based on various external effects. For example, the addition of a resistive heater for electrothermal conversion (''e.g.'' single walled carbon nanotube
A carbon nanotube (CNT) is a tube made of carbon with a diameter in the nanometre range ( nanoscale). They are one of the allotropes of carbon. Two broad classes of carbon nanotubes are recognized:
* ''Single-walled carbon nanotubes'' (''S ...
s) can allow for an on-demand mechanical property switch via an electric current
An electric current is a flow of charged particles, such as electrons or ions, moving through an electrical conductor or space. It is defined as the net rate of flow of electric charge through a surface. The moving particles are called charge c ...
. Alternatively, by adding a filler like graphene oxide, light irradiation can be used for an induced photo-thermal effect allowing for switching of the mechanical properties as a response to light-irradiation. Other interesting nanoparticles for the application in CANs include clay nanosheets, graphene and cellulose
Cellulose is an organic compound with the chemical formula, formula , a polysaccharide consisting of a linear chain of several hundred to many thousands of glycosidic bond, β(1→4) linked glucose, D-glucose units. Cellulose is an important s ...
.
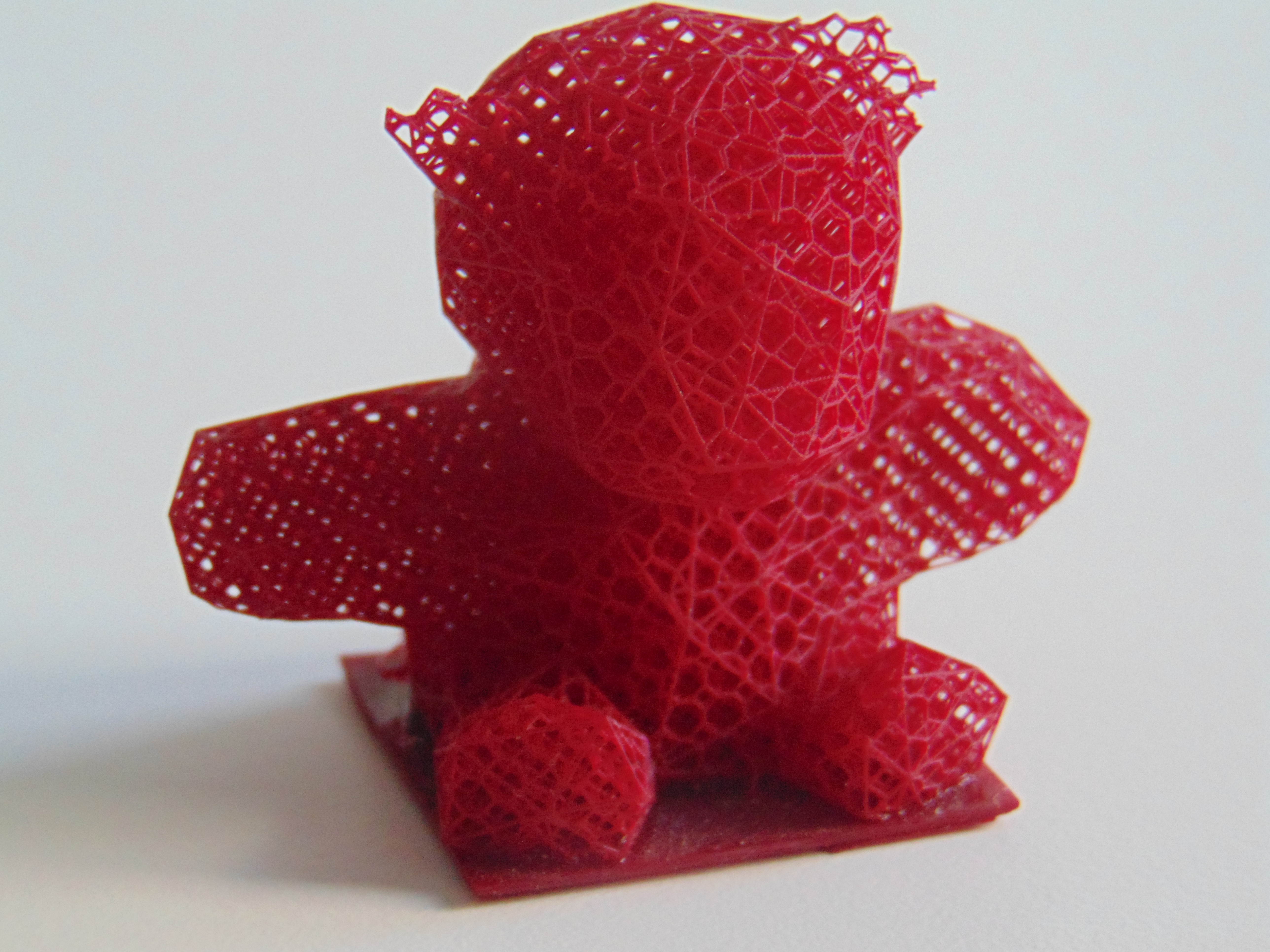
3D printing
 In recent years,
In recent years, 3D printing
3D printing, or additive manufacturing, is the construction of a three-dimensional object from a CAD model or a digital 3D model. It can be done in a variety of processes in which material is deposited, joined or solidified under computer ...
, or additive manufacturing (AM), saw rapid developments as the technique became more and more popular. Currently, plastics are the most common raw material used for 3D printing due to their wide availability, diversity and light weight. The versatility of AM and its significant development resulted in its use for many applications
Application may refer to:
Mathematics and computing
* Application software, computer software designed to help the user to perform specific tasks
** Application layer, an abstraction layer that specifies protocols and interface methods used in a ...
ranging from manufacturing and medical sectors to the custom art and design sector. With the market of 3D printing expected to grow even further in the coming years, the use of CANs as a resource for AM is under investigation as a replacement for traditional thermosets, which could make up 22% of the global market for AM by the end of 2029.
By replacing traditional thermoset ink with CAN-based inks, complicated 3D geometries can still be printed that behave like traditional thermosets with excellent mechanical properties at service conditions, but can later also be recycled into new ink for the next round of 3D printing. One example involved the 3D printing of an epoxy ink which is able to undergo transesterification reactions after printing. During the printing cycle, the ink is first slightly cured before being printed at high temperature into the desired 3D structure, and followed by a second curing step in an oven after printing. The printed epoxy parts can then be recycled by dissolving in ethylene glycol
Ethylene glycol ( IUPAC name: ethane-1,2-diol) is an organic compound (a vicinal diol) with the formula . It is mainly used for two purposes: as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an odo ...
at high temperature and reused as ink in a new printing cycle.
Chemistries used in CANs
Various dynamic chemistries have already been incorporated in CANs; some of the more notable ones includetransesterification
Transesterification is the process of exchanging the organic functional group R″ of an ester with the organic group R' of an alcohol. These reactions are often catalyzed by the addition of an acid or base catalyst. Strong acids catalyze the r ...
, Diels-Alder exchange, imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
metathesis, disulfide
In chemistry, a disulfide (or disulphide in British English) is a compound containing a functional group or the anion. The linkage is also called an SS-bond or sometimes a disulfide bridge and usually derived from two thiol groups.
In inorg ...
exchange, transamination
Transamination is a chemical reaction that transfers an amino group to a ketoacid to form new amino acids.This pathway is responsible for the deamination of most amino acids. This is one of the major degradation pathways which convert essential a ...
of vinylogous urethanes, transcarbamoylation of urethanes, olefin metathesis, and trans-N-alkylation of 1,2,3-triazolium salts.
Sulfur-based CANs
Due to their relatively weakbond dissociation energy
The bond-dissociation energy (BDE, ''D''0, or ''DH°'') is one measure of the strength of a chemical bond . It can be defined as the standard enthalpy change when is cleaved by homolysis to give fragments A and B, which are usually radical ...
(in comparison to C−C bonds and the like), disulfides have been employed in CAN systems in order to allow for dynamic breakage and reformation of crosslinks. With disulfide groups as crosslinks, materials can be produced which are stable at room temperature while also allowing for reversible crosslink dissociation upon heating. The mechanism behind this reaction can be attributed to the cleavage of disulfide linkages (RS−SR) into thiyl radicals (2 RS•), resulting in reprocessability and self-healing characteristics for the bulk material. However, since the bond dissociation energy of the disulfide bond is still fairly high, it is typically necessary to augment the bond with adjacent chemistry that can stabilize the unpaired electron of the intermediate state. As such, studies usually employ aromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugati ...
disulfides or disulfidediamine (RNS−SNR) functional groups to encourage the dynamic dissociation of the S−S bond; these chemistries can result in the bond dissociation energy being reduced to half (or even less) of its prior magnitude.
In practical terms, disulfide-containing CANs are similar to thermosets. Typically, recyclability is restricted to thermoplastic
A thermoplastic, or thermosoftening plastic, is any plastic polymer material that becomes pliable or moldable at a certain elevated temperature and solidifies upon cooling.
Most thermoplastics have a high molecular weight. The polymer chains as ...
materials, as said materials consist of polymer chains which are not bonded to each other at the molecular level; as a result, they can be melted down and reformed (as the addition of thermal energy allows the chains to untangle, move past each other, and adopt new configurations), but this comes at the expense of their physical robustness. Meanwhile, conventional thermosets contain permanent crosslinks which bolster their strength
Strength may refer to:
Personal trait
*Physical strength, as in people or animals
*Character strengths like those listed in the Values in Action Inventory
*The exercise of willpower
Physics
* Mechanical strength, the ability to withstand ...
, toughness
In materials science and metallurgy, toughness is the ability of a material to absorb energy and plastically deform without fracturing.creep resistance, and the like (as the bonding between chains provides resistance to deformation at the macroscopic level), but due to the permanence of said crosslinks, these materials cannot be reprocessed akin to thermoplastics. However, due to the dynamic nature of the crosslinks in disulfide CANs, they can be designed to exhibit the best attributes of both of the aforementioned material types. Studies have shown that disulfide CANs can be reprocessed multiple times with negligible degradation in performance while also exhibiting creep resistance,
glass transition
The glass–liquid transition, or glass transition, is the gradual and Reversible reaction, reversible transition in amorphous solid, amorphous materials (or in amorphous regions within Crystallinity, semicrystalline materials) from a hard and rel ...
, and dynamic modulus
Dynamic modulus (sometimes complex modulusThe Open University (UK), 2000. ''T838 Design and Manufacture with Polymers: Solid properties and design'', page 30. Milton Keynes: The Open University.) is the ratio of stress to strain under ''vibratory ...
values comparable to those observed in similar conventional thermoset systems.
References
{{Reflist Polymers