Copper(II) Chloride on:
[Wikipedia]
[Google]
[Amazon]
Copper(II) chloride, also known as cupric chloride, is an
 Aqueous solutions prepared from copper(II) chloride contain a range of copper(II) complexes depending on
Aqueous solutions prepared from copper(II) chloride contain a range of copper(II) complexes depending on
 This reaction is performed in a polar solvent such as
This reaction is performed in a polar solvent such as  Such compounds are intermediates in the synthesis of BINAP and its derivatives.
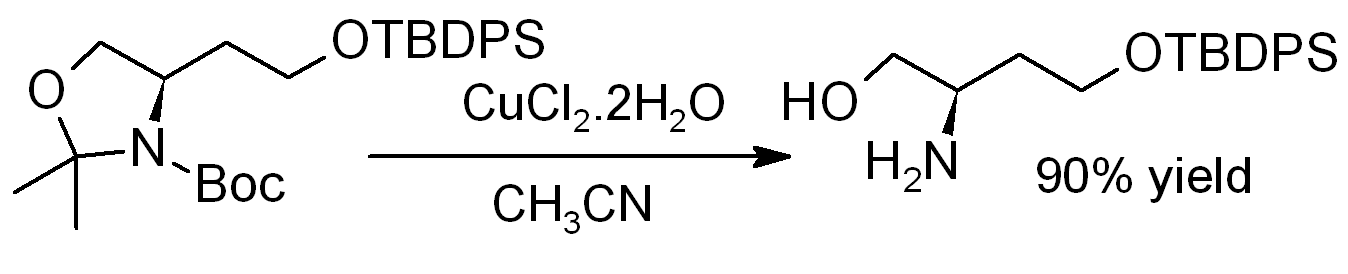
Copper(II) chloride dihydrate promotes the hydrolysis of acetonides, i.e., for deprotection to regenerate diols or aminoalcohols, as in this example (where TBDPS = ''tert''-butyldiphenylsilyl):
:
Such compounds are intermediates in the synthesis of BINAP and its derivatives.
Copper(II) chloride dihydrate promotes the hydrolysis of acetonides, i.e., for deprotection to regenerate diols or aminoalcohols, as in this example (where TBDPS = ''tert''-butyldiphenylsilyl):
: also catalyses the
also catalyses the
 Copper(II) chloride occurs naturally as the very rare anhydrous mineral tolbachite and the dihydrate eriochalcite.Marlene C. Morris, Howard F. McMurdie, Eloise H. Evans, Boris Paretzkin, Harry S. Parker, and Nicolas C. Panagiotopoulos (1981) ''Copper chloride hydrate (eriochalcite)'', in
Copper(II) chloride occurs naturally as the very rare anhydrous mineral tolbachite and the dihydrate eriochalcite.Marlene C. Morris, Howard F. McMurdie, Eloise H. Evans, Boris Paretzkin, Harry S. Parker, and Nicolas C. Panagiotopoulos (1981) ''Copper chloride hydrate (eriochalcite)'', in
Standard X-ray Diffraction Powder Patterns
National Bureau of Standards, Monograph 25, Section 18; page 33. Both are found near fumaroles and in some copper mines. Mixed oxyhydroxide-chlorides like atacamite () are more common, arising among Cu ore beds oxidation zones in arid climates.
Copper Chloride
at ''
Copper (II) Chloride – Description and Pictures
{{Chlorides Copper(II) compounds Chlorides Metal halides Semiconductor materials Coordination complexes Pyrotechnic colorants
inorganic compound
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemistry''.
Inorgan ...
with the chemical formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as pare ...
. The monoclinic
In crystallography, the monoclinic crystal system is one of the seven crystal systems. A crystal system is described by three Vector (geometric), vectors. In the monoclinic system, the crystal is described by vectors of unequal lengths, as in t ...
yellowish-brown anhydrous
A substance is anhydrous if it contains no water. Many processes in chemistry can be impeded by the presence of water; therefore, it is important that water-free reagents and techniques are used. In practice, however, it is very difficult to achie ...
form slowly absorbs moisture to form the orthorhombic blue-green dihydrate , with two water molecules of hydration. It is industrially produced for use as a co-catalyst in the Wacker process.
Both the anhydrous and the dihydrate forms occur naturally as the rare minerals tolbachite and eriochalcite, respectively.
Structure
Anhydrous copper(II) chloride adopts a distorted cadmium iodide structure. In this structure, thecopper
Copper is a chemical element; it has symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orang ...
centers are octahedral. Most copper(II) compounds exhibit distortions from idealized octahedral geometry due to the Jahn-Teller effect, which in this case describes the localization of one d-electron into a molecular orbital that is strongly antibonding with respect to a pair of chloride ligands. In , the copper again adopts a highly distorted octahedral geometry, the Cu(II) centers being surrounded by two water ligands and four chloride ligands, which bridge
A bridge is a structure built to Span (engineering), span a physical obstacle (such as a body of water, valley, road, or railway) without blocking the path underneath. It is constructed for the purpose of providing passage over the obstacle, whi ...
asymmetrically to other Cu centers.
Copper(II) chloride is paramagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, ...
. Of historical interest, was used in the first electron paramagnetic resonance
Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a method for studying materials that have unpaired electrons. The basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but the spin ...
measurements by Yevgeny Zavoisky in 1944.
Properties and reactions
concentration
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', '' molar concentration'', '' number concentration'', ...
, temperature, and the presence of additional chloride ions. These species include the blue color of and the yellow or red color of the halide complexes of the formula .Greenwood, N. N. and Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. p. 1183–1185 .
Hydrolysis
When copper(II) chloride solutions are treated with a base, aprecipitation
In meteorology, precipitation is any product of the condensation of atmospheric water vapor that falls from clouds due to gravitational pull. The main forms of precipitation include drizzle, rain, rain and snow mixed ("sleet" in Commonwe ...
of copper(II) hydroxide
Copper(II) hydroxide is the hydroxide of copper with the chemical formula of Cu(OH)2. It is a pale greenish blue or bluish green solid. Some forms of copper(II) hydroxide are sold as "stabilized" copper(II) hydroxide, although they likely consist ...
occurs:
:
Partial hydrolysis gives dicopper chloride trihydroxide
Dicopper chloride trihydroxide is the compound with chemical formula . It is often referred to as tribasic copper chloride (TBCC), copper trihydroxyl chloride or copper hydroxychloride. This greenish substance is encountered as the minerals ata ...
, , a popular fungicide. When an aqueous solution of copper(II) chloride is left in the air and isn't stabilized by a small amount of acid, it is prone to undergo slight hydrolysis.
Redox and decomposition
Copper(II) chloride is a mildoxidant
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or "Electron acceptor, accepts"/"receives" an electron from a (called the , , or ''electr ...
. It starts to decompose to copper(I) chloride
Copper(I) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula CuCl. The substance is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. Impure samples appear gr ...
and chlorine gas around and is completely decomposed near :
:
The reported melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state of matter, state from solid to liquid. At the melting point the solid and liquid phase (matter), phase exist in Thermodynamic equilib ...
of copper(II) chloride of is a melt of a mixture of copper(I) chloride and copper(II) chloride. The true melting point of can be extrapolated by using the melting points of the mixtures of CuCl and . Copper(II) chloride reacts with several metals to produce copper metal or copper(I) chloride (CuCl) with oxidation of the other metal. To convert copper(II) chloride to copper(I) chloride, it can be convenient to reduce an aqueous solution with sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a colorless gas with a pungent smell that is responsible for the odor of burnt matches. It is r ...
as the reductant:
:
Coordination complexes
reacts with HCl or otherchloride
The term chloride refers to a compound or molecule that contains either a chlorine anion (), which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond (). The pr ...
sources to form complex ions: the red (found in potassium trichloridocuprate(II) ) (it is a dimer in reality, , a couple of tetrahedrons that share an edge), and the green or yellow (found in potassium tetrachloridocuprate(II) ).
:
:
Some of these complexes can be crystallized from aqueous solution, and they adopt a wide variety of structures.
Copper(II) chloride also forms a variety of coordination complexes with ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
s such as ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
, pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom . It is a highly flammable, weak ...
and triphenylphosphine oxide:
: (tetragonal)
: (tetrahedral)
However "soft" ligands such as phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
s (e.g., triphenylphosphine), iodide, and cyanide
In chemistry, cyanide () is an inorganic chemical compound that contains a functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom.
Ionic cyanides contain the cyanide anion . This a ...
as well as some tertiary amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
s induce reduction to give copper(I) complexes.
Preparation
Copper(II) chloride is prepared commercially by the action of chlorination of copper. Copper at red heat (300-400°C) combines directly with chlorine gas, giving (molten) copper(II) chloride. The reaction is veryexothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity (e ...
.
:
A solution of copper(II) chloride is commercially produced by adding chlorine gas to a circulating mixture of hydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
and copper. From this solution, the dihydrate can be produced by evaporation.
Although copper metal itself cannot be oxidized by hydrochloric acid, copper-containing bases such as the hydroxide, oxide
An oxide () is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion (anion bearing a net charge of −2) of oxygen, an O2− ion with oxygen in the oxidation st ...
, or copper(II) carbonate can react to form in an acid-base reaction which can subsequently be heated above to produce the anhydrous derivative.
Once prepared, a solution of may be purified by crystallization
Crystallization is a process that leads to solids with highly organized Atom, atoms or Molecule, molecules, i.e. a crystal. The ordered nature of a crystalline solid can be contrasted with amorphous solids in which atoms or molecules lack regu ...
. A standard method takes the solution mixed in hot dilute hydrochloric acid, and causes the crystals to form by cooling in a calcium chloride
Calcium chloride is an inorganic compound, a Salt (chemistry), salt with the chemical formula . It is a white crystalline solid at room temperature, and it is highly soluble in water. It can be created by neutralising hydrochloric acid with cal ...
() ice bath.S. H. Bertz, E. H. Fairchild, in ''Handbook of Reagents for Organic Synthesis, Volume 1: Reagents, Auxiliaries and Catalysts for C-C Bond Formation'', (R. M. Coates, S. E. Denmark, eds.), pp. 220–223, Wiley, New York, 1738.
There are indirect and rarely used means of using copper ions in solution to form copper(II) chloride. Electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses Direct current, direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of c ...
of aqueous sodium chloride with copper electrodes
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or a gas). In electrochemical cells, electrodes are essential parts that can consist of a variety ...
produces (among other things) a blue-green foam
Foams are two-phase materials science, material systems where a gas is dispersed in a second, non-gaseous material, specifically, in which gas cells are enclosed by a distinct liquid or solid material. Note, this source focuses only on liquid ...
that can be collected and converted to the hydrate. While this is not usually done due to the emission of toxic chlorine gas, and the prevalence of the more general chloralkali process
The chloralkali process (also chlor-alkali and chlor alkali) is an industrial process for the electrolysis of sodium chloride (NaCl) solutions. It is the technology used to produce chlorine and sodium hydroxide (caustic soda), which are commodi ...
, the electrolysis will convert the copper metal to copper ions in solution forming the compound. Indeed, any solution of copper ions can be mixed with hydrochloric acid and made into a copper chloride by removing any other ions.
Uses
Co-catalyst in Wacker process
A major industrial application for copper(II) chloride is as a co-catalyst with palladium(II) chloride in the Wacker process. In this process,ethene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon–carbon double bonds).
Ethy ...
(ethylene) is converted to ethanal (acetaldehyde) using water and air. During the reaction, reduced to Pd, and the serves to re-oxidize this back to . Air can then oxidize the resultant CuCl back to , completing the cycle.
#
#
#
The overall process is:
:
In organic synthesis
Copper(II) chloride has some highly specialized applications in the synthesis of organic compounds. It affects the chlorination of aromatic hydrocarbons—this is often performed in the presence ofaluminium oxide
Aluminium oxide (or aluminium(III) oxide) is a chemical compound of aluminium and oxygen with the chemical formula . It is the most commonly occurring of several Aluminium oxide (compounds), aluminium oxides, and specifically identified as alum ...
. It is able to chlorinate the alpha position of carbonyl
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such a ...
compounds:
: This reaction is performed in a polar solvent such as
This reaction is performed in a polar solvent such as dimethylformamide
Dimethylformamide, DMF is an organic compound with the chemical formula . Its structure is . Commonly abbreviated as DMF (although this initialism is sometimes used for 2,5-dimethylfuran, dimethylfuran, or dimethyl fumarate), this colourless liqui ...
, often in the presence of lithium chloride
Lithium chloride is a chemical compound with the formula Li Cl. The salt is a typical ionic compound (with certain covalent characteristics), although the small size of the Li+ ion gives rise to properties not seen for other alkali metal chlorid ...
, which accelerates the reaction.
, in the presence of oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
, can also oxidize phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (− O H) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, . Phenolic compounds ar ...
. The major product can be directed to give either a quinone
The quinones are a class of organic compounds that are formally "derived from aromatic compounds benzene.html" ;"title="uch as benzene">uch as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with ...
or a coupled product from oxidative dimerization. The latter process provides a high-yield route to 1,1-binaphthol:
: Such compounds are intermediates in the synthesis of BINAP and its derivatives.
Copper(II) chloride dihydrate promotes the hydrolysis of acetonides, i.e., for deprotection to regenerate diols or aminoalcohols, as in this example (where TBDPS = ''tert''-butyldiphenylsilyl):
:
Such compounds are intermediates in the synthesis of BINAP and its derivatives.
Copper(II) chloride dihydrate promotes the hydrolysis of acetonides, i.e., for deprotection to regenerate diols or aminoalcohols, as in this example (where TBDPS = ''tert''-butyldiphenylsilyl):
: also catalyses the
also catalyses the free radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabolic disorders
Metabolism
...
addition of sulfonyl chlorides to alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
s; the alpha-chlorosulfone may then undergo elimination with a base to give a vinyl sulfone product.
Catalyst in production of chlorine
Copper(II) chloride is used as acatalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
in a variety of processes that produce chlorine by oxychlorination. The Deacon process takes place at about 400 to 450 °C in the presence of a copper chloride:
:
Copper(II) chloride catalyzes the chlorination in the production of vinyl chloride
Vinyl chloride is an organochloride with the formula H2C =CHCl. It is also called vinyl chloride monomer (VCM) or chloroethene. It is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride (PVC). Vinyl chloride is a ...
and dichloromethane.
Copper(II) chloride is used in the copper–chlorine cycle where it reacts with steam into copper(II) oxide dichloride and hydrogen chloride and is later recovered in the cycle from the electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses Direct current, direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of c ...
of copper(I) chloride.
Niche uses
Copper(II) chloride is used inpyrotechnics
Pyrotechnics is the science and craft of creating fireworks, but also includes safety matches, oxygen candles, Pyrotechnic fastener, explosive bolts (and other fasteners), parts of automotive airbags, as well as gas-pressure blasting in mining, q ...
as a blue/green coloring agent. In a flame test, copper chlorides, like all copper compounds, emit green-blue light.
In humidity indicator cards (HICs), cobalt-free brown to azure (copper(II) chloride base) HICs can be found on the market. In 1998, the European Community
The European Economic Community (EEC) was a regional organisation created by the Treaty of Rome of 1957,Today the largely rewritten treaty continues in force as the ''Treaty on the functioning of the European Union'', as renamed by the Lisbo ...
classified items containing cobalt(II) chloride of 0.01 to 1% w/w as T (Toxic), with the corresponding R phrase of R49 (may cause cancer if inhaled). Consequently, new cobalt-free humidity indicator cards containing copper have been developed.
Copper(II) chloride is used as a mordant in the textile industry, petroleum
Petroleum, also known as crude oil or simply oil, is a naturally occurring, yellowish-black liquid chemical mixture found in geological formations, consisting mainly of hydrocarbons. The term ''petroleum'' refers both to naturally occurring un ...
sweetener, wood preservative
Wood preservation refers to any method or process, or even technique, used to protect the wood and extend its service life.
Most wood species are susceptible to both biological (''biotic'') and non-biological (''abiotic'') factors that cause d ...
, and water cleaner.
Copper(II) chloride is also used in high school demos
Demos may refer to:
Computing
* DEMOS, a Soviet Unix-like operating system
* DEMOS (ISP), the first internet service provider in the USSR
* Demos Commander, an Orthodox File Manager for Unix-like systems
* Plural for Demo (computer programming ...
, such as reacting with aluminum
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
to create aluminum chloride and copper
Copper is a chemical element; it has symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orang ...
, and learning how to measure moles.
Natural occurrence
 Copper(II) chloride occurs naturally as the very rare anhydrous mineral tolbachite and the dihydrate eriochalcite.Marlene C. Morris, Howard F. McMurdie, Eloise H. Evans, Boris Paretzkin, Harry S. Parker, and Nicolas C. Panagiotopoulos (1981) ''Copper chloride hydrate (eriochalcite)'', in
Copper(II) chloride occurs naturally as the very rare anhydrous mineral tolbachite and the dihydrate eriochalcite.Marlene C. Morris, Howard F. McMurdie, Eloise H. Evans, Boris Paretzkin, Harry S. Parker, and Nicolas C. Panagiotopoulos (1981) ''Copper chloride hydrate (eriochalcite)'', in Standard X-ray Diffraction Powder Patterns
National Bureau of Standards, Monograph 25, Section 18; page 33. Both are found near fumaroles and in some copper mines. Mixed oxyhydroxide-chlorides like atacamite () are more common, arising among Cu ore beds oxidation zones in arid climates.
Safety and biological impact
Copper(II) chloride can be toxic. Only concentrations below 1.3 ppm of aqueous copper ions are allowed in drinking water by the US Environmental Protection Agency. If copper chloride is absorbed, it results in headache, diarrhea, a drop inblood pressure
Blood pressure (BP) is the pressure of Circulatory system, circulating blood against the walls of blood vessels. Most of this pressure results from the heart pumping blood through the circulatory system. When used without qualification, the term ...
, and fever. Ingestion of large amounts may induce copper poisoning, CNS disorders, and haemolysis.
Copper(II) chloride has been demonstrated to cause chromosomal aberrations and mitotic cycle disturbances within A. cepa (onion) cells. Such cellular disturbances lead to genotoxicity
Genotoxicity is the chemical property, property of chemical agents that damage the genetic information within a cell causing mutations, which may lead to cancer. While genotoxicity is often confused with mutagenicity, all mutagens are genotoxic, bu ...
. Copper(II) chloride has also been studied as a harmful environmental pollutant. Often present in irrigation-grade water, it can negatively affect water and soil microbes. Specifically, denitrifying bacteria were found to be very sensitive to the presence of copper(II) chloride. At a concentration of 0.95 mg/L, copper(II) chloride was found to cause a 50% inhibition (IC50) of the metabolic activity of denitrifying microbes.
See also
*Copper(I) chloride
Copper(I) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula CuCl. The substance is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. Impure samples appear gr ...
References
Further reading
* * * ''The Merck Index'', 7th edition, Merck & Co, Rahway, New Jersey, USA, 1960. * D. Nicholls, ''Complexes and First-Row Transition Elements'', Macmillan Press, London, 1973. * A. F. Wells, Structural Inorganic Chemistry'', 5th ed., Oxford University Press, Oxford, UK, 1984. * J. March, ''Advanced Organic Chemistry'', 4th ed., p. 723, Wiley, New York, 1992. * ''Fieser & Fieser Reagents for Organic Synthesis'' Volume 5, p158, Wiley, New York, 1975. *External links
Copper Chloride
at ''
The Periodic Table of Videos
''Periodic Videos'' (also known as ''The Periodic Table of Videos'') is a video project and YouTube channel on chemistry. It consists of a series of videos about chemical elements and the periodic table, with additional videos on other topics i ...
'' (University of Nottingham)
Copper (II) Chloride – Description and Pictures
{{Chlorides Copper(II) compounds Chlorides Metal halides Semiconductor materials Coordination complexes Pyrotechnic colorants