Colloid Osmotic Pressure on:
[Wikipedia]
[Google]
[Amazon]
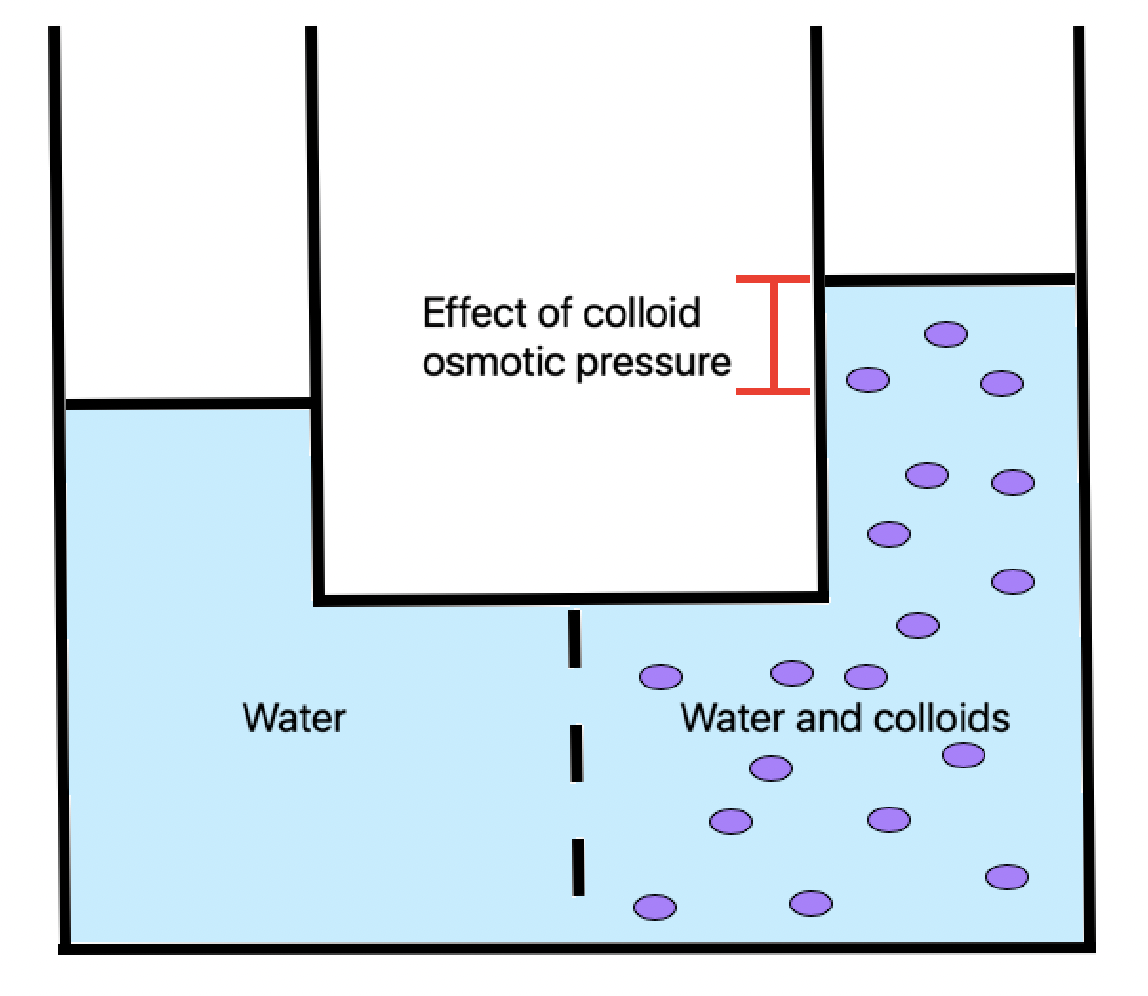 Oncotic pressure, or colloid osmotic-pressure, is a type of
Oncotic pressure, or colloid osmotic-pressure, is a type of
Overview at cvphysiology.com
Physiology
 Oncotic pressure, or colloid osmotic-pressure, is a type of
Oncotic pressure, or colloid osmotic-pressure, is a type of osmotic pressure
Osmotic pressure is the minimum pressure which needs to be applied to a Solution (chemistry), solution to prevent the inward flow of its pure solvent across a semipermeable membrane.
It is also defined as the measure of the tendency of a soluti ...
induced by the plasma proteins, notably albumin, in a blood vessel's plasma (or any other body fluid
Body fluids, bodily fluids, or biofluids, sometimes body liquids, are liquids within the Body (biology), body of an organism. In lean healthy adult men, the total body water is about 60% (60–67%) of the total Human body weight, body weight; it ...
such as blood and lymph) that causes a pull on fluid back into the capillary.
It has an effect opposing both the hydrostatic blood pressure
Blood pressure (BP) is the pressure of Circulatory system, circulating blood against the walls of blood vessels. Most of this pressure results from the heart pumping blood through the circulatory system. When used without qualification, the term ...
, which pushes water and small molecules out of the blood into the interstitial spaces at the arterial end of capillaries, and the interstitial colloidal osmotic pressure. These interacting factors determine the partitioning of extracellular water between the blood plasma and the extravascular space.
Oncotic pressure strongly affects the physiological function of the circulatory system. It is suspected to have a major effect on the pressure across the glomerular filter. However, this concept has been strongly criticised and attention has shifted to the impact of the intravascular glycocalyx layer as the major player.
Etymology
The word 'oncotic' by definition is termed as 'pertaining to swelling', indicating the effect of oncotic imbalance on the swelling of tissues. The word itself is derived from onco- and -ic; 'onco-' meaning 'pertaining to mass or tumors' and '-ic', which forms an adjective.Description
Throughout the body, dissolved compounds have an osmotic pressure. Because large plasma proteins cannot easily cross through thecapillary
A capillary is a small blood vessel, from 5 to 10 micrometres in diameter, and is part of the microcirculation system. Capillaries are microvessels and the smallest blood vessels in the body. They are composed of only the tunica intima (the inn ...
walls, their effect on the osmotic pressure of the capillary interiors will, to some extent, balance out the tendency for fluid to leak out of the capillaries. In other words, the oncotic pressure tends to pull fluid into the capillaries. In conditions where plasma proteins are reduced, e.g. from being lost in the urine
Urine is a liquid by-product of metabolism in humans and many other animals. In placental mammals, urine flows from the Kidney (vertebrates), kidneys through the ureters to the urinary bladder and exits the urethra through the penile meatus (mal ...
( proteinuria), there will be a reduction in oncotic pressure and an increase in filtration across the capillary, resulting in excess fluid buildup in the tissues ( edema).
The large majority of oncotic pressure in capillaries is generated by the presence of high quantities of albumin, a protein that constitutes approximately 80% of the total oncotic pressure exerted by blood plasma on interstitial fluid . The total oncotic pressure of an average capillary is about 28 mmHg with albumin contributing approximately 22 mmHg of this oncotic pressure, despite only representing 50% of all protein in blood plasma at 35-50 g/L. Because blood proteins cannot escape through capillary endothelium, oncotic pressure of capillary beds tends to draw water into the vessels. It is necessary to understand the oncotic pressure as a balance; because the blood proteins reduce interior permeability, less plasma fluid can exit the vessel.
Oncotic pressure is represented by the symbol Π or π in the Starling equation and elsewhere. The Starling equation in particular describes filtration in volume/s () by relating oncotic pressure () to capillary hydrostatic pressure (), interstitial fluid hydrostatic pressure (), and interstitial fluid oncotic pressure (), as well as several descriptive coefficients, as shown below:
At the arteriolar end of the capillary, blood pressure starts at about 36 mm Hg and decreases to around 15 mm Hg at the venous end, with oncotic pressure at a stable 25–28 mm Hg. Within the capillary, reabsorption due to this venous pressure difference is estimated to be around 90% that of the filtered fluid, with the extra 10% being returned via lymphatics in order to maintain stable blood volume.
Physiological impact
In tissues, physiological disruption can arise with decreased oncotic pressure, which can be determined using blood tests for protein concentration. Decreased colloidal osmotic pressure, most notably seen in hypoalbuminemia, can cause edema and decrease in blood volume as fluid is not reabsorbed into the bloodstream. Colloid pressure in these cases can be lost due to a number of different factors, but primarily decreased colloid production or increased loss of colloids through glomerular filtration. This low pressure often correlates with poor surgical outcomes. In the clinical setting, there are two types of fluids that are used for intravenous drips: crystalloids andcolloid
A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others exte ...
s. Crystalloids are aqueous solution
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, also known as sodium chloride (NaCl), in water ...
s of mineral salts or other water-soluble molecules. Colloids contain larger insoluble molecules, such as gelatin. There is some debate concerning the advantages and disadvantages of using biological vs. synthetic colloid solutions. Oncotic pressure values are approximately 290 mOsm per kg of water, which slightly differs from the osmotic pressure of the blood that has values approximating 300 mOsm /L. These colloidal solutions are typically used to remedy low colloid concentration, such as in hypoalbuminemia, but is also suspected to assist in injuries that typically increase fluid loss, such as burns.
References
{{reflistExternal links
Overview at cvphysiology.com
Physiology