coiled-coil on:
[Wikipedia]
[Google]
[Amazon]
A coiled coil is a
 The general problem of deciding on the folded structure of a protein when given the amino acid sequence (the so-called protein folding problem) has only been solved partially. However, the coiled coil is one of a relatively small number of folding motifs for which the relationships between the sequence and the final folded structure are comparatively well understood.
Harbury ''et al.'' performed a landmark study using an archetypal coiled coil, GCN4, in which rules that govern the way that peptide sequence affects the oligomeric state (that is, the number of alpha-helices in the final assembly) were established.
The GCN4 coiled coil is a 31-amino-acid (which equates to just over four ''heptads'') parallel, dimeric (i.e., consisting of two alpha-helices) coiled coil and has a repeated
The general problem of deciding on the folded structure of a protein when given the amino acid sequence (the so-called protein folding problem) has only been solved partially. However, the coiled coil is one of a relatively small number of folding motifs for which the relationships between the sequence and the final folded structure are comparatively well understood.
Harbury ''et al.'' performed a landmark study using an archetypal coiled coil, GCN4, in which rules that govern the way that peptide sequence affects the oligomeric state (that is, the number of alpha-helices in the final assembly) were established.
The GCN4 coiled coil is a 31-amino-acid (which equates to just over four ''heptads'') parallel, dimeric (i.e., consisting of two alpha-helices) coiled coil and has a repeated
 Coiled-coil motifs have been experimented on as possible building block for nanostructures, in part because of their simple design and wide range of function based primarily on facilitating protein-protein interaction. Simple guidelines for
Coiled-coil motifs have been experimented on as possible building block for nanostructures, in part because of their simple design and wide range of function based primarily on facilitating protein-protein interaction. Simple guidelines for
Coiled-coil domains of keratins
Paircoil2
Paircoil
bCIPA
Estimates Tm values for coiled coil pairs
bCIPA library screen
Screens a library of sequences against a single defined target and estimates Tm values for all coiled coils pairs.
bCIPA Interactome Screen
Screens all interactions between a selection of defined sequences and estimates Tm values for all coiled coil pairs.
STRAP
contains an algorithm to predict coiled-coils from AA-sequences.
PrOCoil
predicts the oligomerization of coiled coil proteins and visualizes the contribution of each individual amino acid to the overall oligomeric tendency.
DrawCoil
creates helical wheel diagrams for coiled coils of any oligomerization state and orientation.
Spiricoil
uses protein domain annotation to predict coiled coil presence and oligormeric state for all completely sequenced organisms
CC+
is a
SUPERFAMILY
protein domain annotation for all completely sequenced organisms based on the expertly curated
structural motif
In a chain-like biological molecule, such as a protein or nucleic acid, a structural motif is a common three-dimensional structure that appears in a variety of different, evolutionarily unrelated molecules. A structural motif does not have t ...
in proteins
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, re ...
in which two to seven alpha-helices are coiled together like the strands of a rope. ( Dimers and trimers are the most common types.) They have been found in roughly 5-10% of proteins and have a variety of functions. They are one of the most widespread motifs found in protein-protein interactions. To aid protein study, several tools have been developed to predict coiled-coils in protein structures. Many coiled coil-type proteins are involved in important biological functions, such as the regulation of gene expression
Gene expression is the process (including its Regulation of gene expression, regulation) by which information from a gene is used in the synthesis of a functional gene product that enables it to produce end products, proteins or non-coding RNA, ...
— e.g., transcription factors
In molecular biology, a transcription factor (TF) (or sequence-specific DNA-binding factor) is a protein that controls the rate of transcription of genetic information from DNA to messenger RNA, by binding to a specific DNA sequence. The fun ...
. Notable examples are the oncoproteins c-Fos and c-Jun, as well as the muscle protein tropomyosin
Tropomyosin is a two-stranded alpha-helical, coiled coil protein found in many animal and fungal cells. In animals, it is an important component of the muscular system which works in conjunction with troponin to regulate muscle contraction. It ...
.
Discovery
The possibility of coiled coils for α-keratin
Keratin () is one of a family of structural fibrous proteins also known as ''scleroproteins''. It is the key structural material making up Scale (anatomy), scales, hair, Nail (anatomy), nails, feathers, horn (anatomy), horns, claws, Hoof, hoove ...
was initially somewhat controversial. Linus Pauling
Linus Carl Pauling ( ; February 28, 1901August 19, 1994) was an American chemist and peace activist. He published more than 1,200 papers and books, of which about 850 dealt with scientific topics. ''New Scientist'' called him one of the 20 gre ...
and Francis Crick
Francis Harry Compton Crick (8 June 1916 – 28 July 2004) was an English molecular biologist, biophysicist, and neuroscientist. He, James Watson, Rosalind Franklin, and Maurice Wilkins played crucial roles in deciphering the Nucleic acid doub ...
independently came to the conclusion that this was possible at about the same time. In the summer of 1952, Pauling visited the laboratory in England
England is a Countries of the United Kingdom, country that is part of the United Kingdom. It is located on the island of Great Britain, of which it covers about 62%, and List of islands of England, more than 100 smaller adjacent islands. It ...
where Crick worked. Pauling and Crick met and spoke about various topics; at one point, Crick asked whether Pauling had considered ''coiled coils'' (a term Crick came up with), to which Pauling said he had. Upon returning to the United States, Pauling resumed research on the topic. He concluded that coiled coils exist, and submitted a lengthy manuscript to the journal ''Nature
Nature is an inherent character or constitution, particularly of the Ecosphere (planetary), ecosphere or the universe as a whole. In this general sense nature refers to the Scientific law, laws, elements and phenomenon, phenomena of the physic ...
'' in October. Pauling's son Peter Pauling worked at the same lab as Crick, and mentioned the report to him. Crick believed that Pauling had stolen his idea, and submitted a shorter note to ''Nature'' a few days after Pauling's manuscript arrived. Eventually, after some controversy and frequent correspondences, Crick's lab declared that the idea had been reached independently by both researchers, and that no intellectual theft had occurred. In his note (which was published first due to its shorter length), Crick proposed the ''coiled coil'' and as well as mathematical methods for determining their structure.
Remarkably, this was soon after the structure of the alpha helix
An alpha helix (or α-helix) is a sequence of amino acids in a protein that are twisted into a coil (a helix).
The alpha helix is the most common structural arrangement in the Protein secondary structure, secondary structure of proteins. It is al ...
was suggested in 1951 by Linus Pauling
Linus Carl Pauling ( ; February 28, 1901August 19, 1994) was an American chemist and peace activist. He published more than 1,200 papers and books, of which about 850 dealt with scientific topics. ''New Scientist'' called him one of the 20 gre ...
and coworkers.
These studies were published in the absence of knowledge of a keratin sequence. The first keratin sequences were determined by Hanukoglu and Fuchs in 1982.
Based on sequence and secondary structure prediction analyses identified the coiled-coil domains of keratins. These models have been confirmed by structural analyses of coiled-coil domains of keratins.
Molecular structure
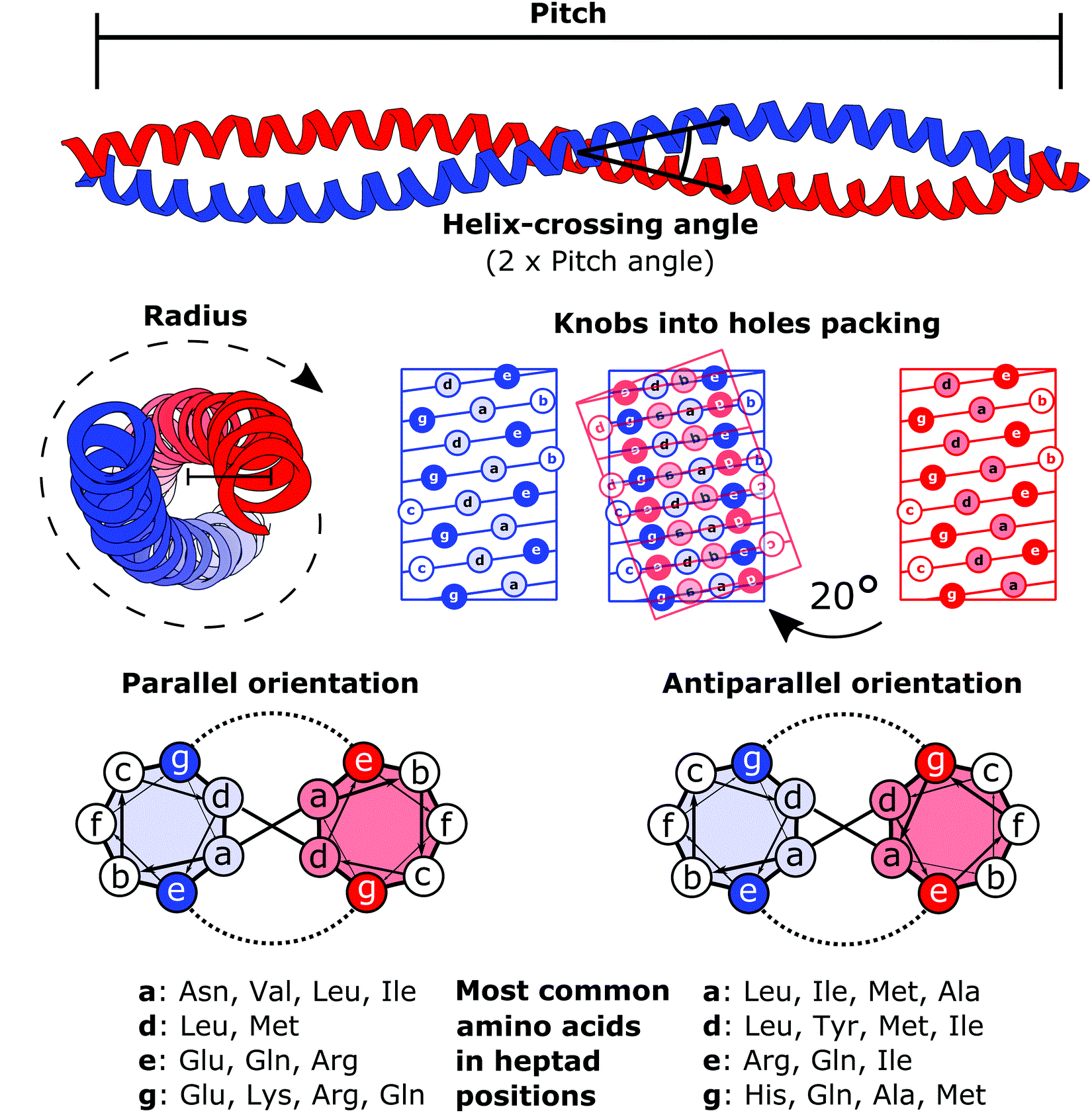
Coiled coils usually contain a repeated pattern, ''hxxhcxc'', of hydrophobic (''h'') and charged (''c'') amino-acid residues, referred to as a heptad repeat. The positions in the heptad repeat are usually labeled ''abcdefg'', where ''a'' and ''d'' are the hydrophobic positions, often being occupied byisoleucine
Isoleucine (symbol Ile or I) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), an α-carboxylic acid group (which is in the depro ...
, leucine
Leucine (symbol Leu or L) is an essential amino acid that is used in the biosynthesis of proteins. Leucine is an α-amino acid, meaning it contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α-Car ...
, or valine
Valine (symbol Val or V) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α- amino group (which is in the protonated −NH3+ form under biological conditions), an α- carboxylic acid group (which is in the deproton ...
. Folding a sequence with this repeating pattern into an alpha-helical secondary structure
Protein secondary structure is the local spatial conformation of the polypeptide backbone excluding the side chains. The two most common Protein structure#Secondary structure, secondary structural elements are alpha helix, alpha helices and beta ...
causes the hydrophobic residues to be presented as a 'stripe' that coils gently around the helix in left-handed fashion, forming an amphipathic structure. The most favorable way for two such helices to arrange themselves in the water-filled environment of the cytoplasm
The cytoplasm describes all the material within a eukaryotic or prokaryotic cell, enclosed by the cell membrane, including the organelles and excluding the nucleus in eukaryotic cells. The material inside the nucleus of a eukaryotic cell a ...
is to wrap the hydrophobic strands against each other sandwiched between the hydrophilic
A hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water.Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon'' Oxford: Clarendon Press.
In contrast, hydrophobes are n ...
amino acids. Thus, it is the burial of hydrophobic surfaces that provides the thermodynamic
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of th ...
driving force for the oligomerization. The packing in a coiled-coil interface is exceptionally tight, with almost complete van der Waals contact between the side-chains of the ''a'' and ''d'' residues. This tight packing was originally predicted by Francis Crick
Francis Harry Compton Crick (8 June 1916 – 28 July 2004) was an English molecular biologist, biophysicist, and neuroscientist. He, James Watson, Rosalind Franklin, and Maurice Wilkins played crucial roles in deciphering the Nucleic acid doub ...
in 1952 and is referred to as knobs into holes packing.
The α-helices may be parallel or anti-parallel, and usually adopt a ''left-handed'' super-coil (Figure 1). Although disfavored, a few ''right-handed'' coiled coils have also been observed in nature and in designed proteins.
Biological roles
As coiled-coil domains are common among a significant amount of proteins in a wide variety of protein families, they help proteins fulfill various functions in the cell. Their primary feature is to facilitate protein-protein interaction and keep proteins or domains interlocked. This feature corresponds to several subfunctions, including membrane fusion, molecular spacing, oligomerization tags, vesicle movement, aid in movement proteins, cell structure, and more.Membrane fusion
A coiled coil domain plays a role inhuman immunodeficiency virus type 1
The subtypes of HIV include two main subtypes, known as HIV type 1 (HIV-1) and HIV type 2 (HIV-2). These subtypes have distinct genetic differences and are associated with different epidemiological patterns and clinical characteristics.
HIV-1 e ...
(HIV-1) infection. Viral entry into CD4-positive cells commences when three subunits of a glycoprotein 120 ( gp120) bind to CD4 receptor and a coreceptor. Glycoprotein gp120 is closely associated with a trimer of gp41 via van der Waals interactions. Eventually, the gp41 N-terminal fusion peptide sequence anchors into the host cell. A spring-loaded mechanism is responsible for bringing the viral and cell membranes in close enough proximity that they will fuse. The origin of the spring-loaded mechanism lies within the exposed gp41, which contains two consecutive heptad repeats (HR1 and HR2) following the fusion peptide at the N terminus of the protein. HR1 forms a parallel, trimeric coiled coil onto which HR2 region coils, forming the trimer-of-hairpins (or six-helix bundle) structure, thereby facilitating membrane fusion through bringing the membranes close to each other. The virus then enters the cell and begins its replication. Recently, inhibitors derived from HR2 such as Fuzeon (DP178, T-20) that bind to the HR1 region on gp41 have been developed. However, peptides derived from HR1 have little viral inhibition efficacy due to the propensity for these peptides to aggregate in solution. Chimeras of these HR1-derived peptides with GCN4 leucine zippers have been developed and have shown to be more active than Fuzeon. Human immunodeficiency virus type 2 has a membrane envelope glycoprotein with similar structure to HIV-1 gp41, but containing substitutions for a glycine amino acid residue in the coiled coil domain that may impact trimer stability.
The proteins SNAP-25, synaptobrevin, and syntaxin-1 have alpha-helices which interact with each other to form a coiled-coil SNARE complex. Zippering the domains together provides the necessary energy for vesicle fusion to occur.
Molecular spacers
The coiled-coil motif may also act as a spacer between two objects within a cell. The lengths of these molecular spacer coiled-coil domains are highly conserved. The purpose of these molecular spacers may be to separate protein domains, thus keeping them from interacting, or to separate vesicles within the cell to mediate vesicle transport. An example of this first purpose is Omp‐α found in '' T. maritima''. Other proteins keep vesicles apart, such as p115, giantin, and GM130 which interact with each other via coiled-coil motifs and act as a tether between the Golgi and a nearby vesicle. The family of proteins related to this activity of tethering vesicles to the Golgi are known as golgins. Finally, there are several proteins with coiled-coil domains involved in thekinetochore
A kinetochore (, ) is a flared oblique-shaped protein structure associated with duplicated chromatids in eukaryotic cells where the spindle fibers, which can be thought of as the ropes pulling chromosomes apart, attach during cell division to ...
, which keeps chromosomes
A chromosome is a package of DNA containing part or all of the genetic material of an organism. In most chromosomes, the very long thin DNA fibers are coated with nucleosome-forming packaging proteins; in eukaryotic cells, the most importa ...
separated during cell division
Cell division is the process by which a parent cell (biology), cell divides into two daughter cells. Cell division usually occurs as part of a larger cell cycle in which the cell grows and replicates its chromosome(s) before dividing. In eukar ...
. These proteins include Ndc-80, and Nuf2p. Related proteins interact with microtubules
Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nm and have an inner diameter between 11 an ...
during cell division, of which mutation leads to cell death.
As oligomerization tags
Because of their specific interaction coiled coils can be used as "tags" to stabilize or enforce a specific oligomerization state. A coiled coil interaction has been observed to drive the oligomerization of the BBS2 and BBS7 subunits of the BBSome. Because coiled-coils generally interact with other coiled coils, they are found in proteins which are required to form dimers or tetramers with more copies of themselves. Because of their ability in driving protein oligomerization, they have also been studied in their use in forming synthetic nanostructures.Design
 The general problem of deciding on the folded structure of a protein when given the amino acid sequence (the so-called protein folding problem) has only been solved partially. However, the coiled coil is one of a relatively small number of folding motifs for which the relationships between the sequence and the final folded structure are comparatively well understood.
Harbury ''et al.'' performed a landmark study using an archetypal coiled coil, GCN4, in which rules that govern the way that peptide sequence affects the oligomeric state (that is, the number of alpha-helices in the final assembly) were established.
The GCN4 coiled coil is a 31-amino-acid (which equates to just over four ''heptads'') parallel, dimeric (i.e., consisting of two alpha-helices) coiled coil and has a repeated
The general problem of deciding on the folded structure of a protein when given the amino acid sequence (the so-called protein folding problem) has only been solved partially. However, the coiled coil is one of a relatively small number of folding motifs for which the relationships between the sequence and the final folded structure are comparatively well understood.
Harbury ''et al.'' performed a landmark study using an archetypal coiled coil, GCN4, in which rules that govern the way that peptide sequence affects the oligomeric state (that is, the number of alpha-helices in the final assembly) were established.
The GCN4 coiled coil is a 31-amino-acid (which equates to just over four ''heptads'') parallel, dimeric (i.e., consisting of two alpha-helices) coiled coil and has a repeated isoleucine
Isoleucine (symbol Ile or I) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), an α-carboxylic acid group (which is in the depro ...
(or I, in single-letter code) and leucine
Leucine (symbol Leu or L) is an essential amino acid that is used in the biosynthesis of proteins. Leucine is an α-amino acid, meaning it contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α-Car ...
(L) at the ''a'' and ''d'' positions, respectively, and forms a dimeric coiled coil. When the amino acids in the ''a'' and ''d'' positions were changed from I at ''a'' and L at ''d'' to I at ''a'' and I at ''d'', a trimeric (three alpha-helices) coiled coil was formed. Furthermore, switching the positions of L to ''a'' and I to ''d'' resulted in the formation of a tetrameric (four alpha-helices) coiled coil. These represent a set of rules for the determination of coiled coil oligomeric states and allows scientists to effectively "dial-in" the oligomerization behavior. Another aspect of coiled coil assembly that is relatively well understood, at least in the case of dimeric coiled coils, is that placing a polar residue (in particular asparagine
Asparagine (symbol Asn or N) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), an α-carboxylic acid group (which is in the depro ...
, N) at opposing ''a'' positions forces parallel assembly of the coiled coil. This effect is due to a self-complementary hydrogen bond
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, Covalent bond, covalently b ...
ing between these residues, which would go unsatisfied if an N were paired with, for instance, an L on the opposing helix.
It was recently demonstrated by Peacock, Pikramenou and co-workers that coiled coils may be self-assembled using lanthanide(III) ions as a template, thus producing novel imaging agents.
Biomedical applications
 Coiled-coil motifs have been experimented on as possible building block for nanostructures, in part because of their simple design and wide range of function based primarily on facilitating protein-protein interaction. Simple guidelines for
Coiled-coil motifs have been experimented on as possible building block for nanostructures, in part because of their simple design and wide range of function based primarily on facilitating protein-protein interaction. Simple guidelines for de novo synthesis
In chemistry, ''de novo'' synthesis () is the synthesis of complex molecules from simple molecules such as sugars or amino acids, as opposed to recycling after partial degradation. For example, nucleotides are not needed in the diet as they can ...
of new proteins containing coiled-coil domains have led to many applications being hypothesized, including drug delivery, regenerating tissue, protein origami, and much more. In regards to drug delivery, coiled-coil domains would help overcome some of the hazards of chemotherapeutic drugs, by keeping them from leaking into healthy tissue as they are transported to their target. Coiled-coil domains can be made to bind to specific proteins or cell surface markers, allowing for more precise targeting in drug delivery. Other functions would be to help store and transport drugs within the body that would otherwise degrade rapidly, by creating nanotubes and other structure svia the interlocking of coiled-coil motifs. By utilizing the function of oligomerization of proteins via coiled-coil domains, antigen display can be amplified in vaccines, increasing their effectiveness.
The oligomerization of coiled-coil motifs allows for the creation of protein origami and protein building blocks. Metal-ligand interactions, covalent bonds, and ionic interactions have been studied to manipulate possible coiled-coil interactions in this field of study. Several different nanostructures can be made by combining coiled-coil motifs such that they are self-assembling building blocks. However, several difficulties remain with stability. Using peptides with coiled-coil motifs for scaffolding has made it easier to create 3D structures for cell culturing. 3D hydrogels can be made with these peptides, and then cells may be loaded into the matrix. This has applications in the study of tissue, tissue engineering, and more.
References
Further reading
* * * * * * * * *External links
Coiled-coil domains of keratins
Coiled-coil related software
Prediction, detection, and visualization
* *Paircoil2
Paircoil
bCIPA
Estimates Tm values for coiled coil pairs
bCIPA library screen
Screens a library of sequences against a single defined target and estimates Tm values for all coiled coils pairs.
bCIPA Interactome Screen
Screens all interactions between a selection of defined sequences and estimates Tm values for all coiled coil pairs.
STRAP
contains an algorithm to predict coiled-coils from AA-sequences.
PrOCoil
predicts the oligomerization of coiled coil proteins and visualizes the contribution of each individual amino acid to the overall oligomeric tendency.
DrawCoil
creates helical wheel diagrams for coiled coils of any oligomerization state and orientation.
Databases
Spiricoil
uses protein domain annotation to predict coiled coil presence and oligormeric state for all completely sequenced organisms
CC+
is a
relational database
A relational database (RDB) is a database based on the relational model of data, as proposed by E. F. Codd in 1970.
A Relational Database Management System (RDBMS) is a type of database management system that stores data in a structured for ...
of coiled coils found in the PDB
SUPERFAMILY
protein domain annotation for all completely sequenced organisms based on the expertly curated
SCOP
A ( or ) was a poet as represented in Old English poetry. The scop is the Old English counterpart of the Old Norse ', with the important difference that "skald" was applied to historical persons, and scop is used, for the most part, to designat ...
coiled coil class
{{Protein secondary structure
Protein folds