Clark Electrode on:
[Wikipedia]
[Google]
[Amazon]
 The Clark electrode is an
The Clark electrode is an
Clark-type Sensors Explained
The Gas Detector Encyclopedia, Edaphic Scientific Knowledge Base
Clark Oxygen Electrode, precursor to today's modern biosensors
- broken link {{DEFAULTSORT:Clark Electrode Electrodes Gas sensors
 The Clark electrode is an
The Clark electrode is an electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or a gas). In electrochemical cells, electrodes are essential parts that can consist of a varie ...
that measures ambient oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
partial pressure
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal g ...
in a liquid using a catalytic platinum
Platinum is a chemical element; it has Symbol (chemistry), symbol Pt and atomic number 78. It is a density, dense, malleable, ductility, ductile, highly unreactive, precious metal, precious, silverish-white transition metal. Its name origina ...
surface according to the net reaction:
: O2 + 4 e− + 4 H+ → 2 H2O
It improves on a bare platinum electrode by use of a membrane to reduce fouling and metal plating onto the platinum.
History
Leland Clark (Professor of Chemistry,Antioch College
Antioch College is a Private college, private Liberal arts colleges in the United States, liberal arts college in Yellow Springs, Ohio, United States. It was founded in 1850 by the Christian Connection and began operating in 1852 as a non-secta ...
, Yellow Springs, Ohio
Yellow Springs is a Village (Ohio), village in northern Greene County, Ohio, United States. The population was 3,697 at the United States Census, 2020, 2020 census. It is part of the Greater Dayton, Dayton metropolitan area and is home to Antioch ...
, and Fels Research Institute, Yellow Springs, Ohio) had developed the first bubble oxygenator for use in cardiac surgery. However, when he came to publish his results, his article was refused by the editor since the oxygen tension in the blood coming out from the device could not be measured. This motivated Clark to develop the oxygen electrode.
The electrode, when implanted in vivo
Studies that are ''in vivo'' (Latin for "within the living"; often not italicized in English) are those in which the effects of various biological entities are tested on whole, living organisms or cells, usually animals, including humans, an ...
, will reduce oxygen and thus required stirring in order to maintain an equilibrium with the environment. Severinghaus improved the design by adding a stirred cuvette in a thermostat. A discrepancy between the measured partial pressure of oxygen (pO2) between blood samples and gaseous mixtures of identical pO2, meant that the modified electrode required calibration; consequently a microtonometer was added to the water thermostat.
Mechanism of action
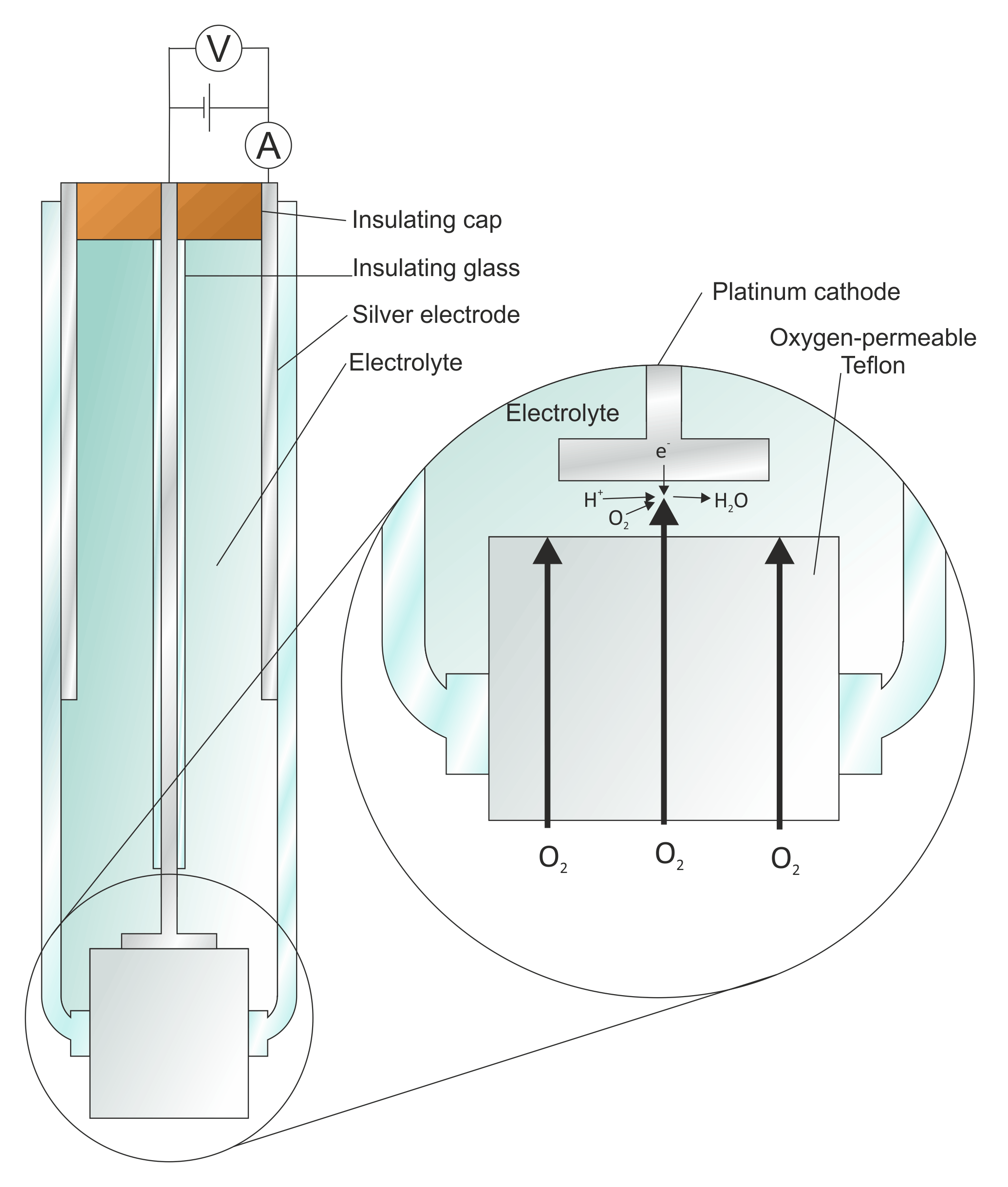
The electrode compartment is isolated from the reaction chamber by a thinTeflon
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene, and has numerous applications because it is chemically inert. The commonly known brand name of PTFE-based composition is Teflon by Chemours, a spin-off from ...
membrane
A membrane is a selective barrier; it allows some things to pass through but stops others. Such things may be molecules, ions, or other small particles. Membranes can be generally classified into synthetic membranes and biological membranes. Bi ...
; the membrane is permeable to molecular oxygen and allows this gas to reach the cathode, where it is electrolytically reduced.
The above reaction requires a steady stream of electrons to the cathode, which depends on the rate at which oxygen can reach the electrode surface. Increasing the voltage applied (between the Pt electrode and a second Ag electrode) will increase the rate of electrocatalysis. Clark affixed an oxygen permselective membrane over the Pt electrode. This limits the diffusion rate of oxygen to the Pt electrode.
Above a certain voltage, the current plateaus and increasing the potential any further does not result in a higher rate of electrocatalysis of the reaction. At this point, the reaction is diffusion-limited and depends only on the permeability properties of the membrane (which is ideally well characterized, the electrode being calibrated against known standard solutions) and by the oxygen gas concentration, which is the measured quantity.
Applications
The Clark oxygen electrode laid the basis for the first glucosebiosensor
A biosensor is an analytical device, used for the detection of a chemical substance, that combines a biological component with a physicochemical detector.
The ''sensitive biological element'', e.g. tissue, microorganisms, organelles, cell rece ...
(in fact the first biosensor of any type), invented by Clark and Lyons in 1962.
This sensor used a single Clark oxygen electrode coupled with a counter-electrode. As with the Clark electrode, a permselective membrane covers the Pt electrode. Now, however, the membrane is impregnated with immobilized glucose oxidase (GOx). The GOx will consume some of the oxygen as it diffuses towards the PT electrode, incorporating it into H2O2 and gluconic acid. The rate of reaction current is limited by the diffusion of both glucose and oxygen. This diffusion can be well characterized for a membrane for both the oxygen and glucose, leaving as the only variable the oxygen and glucose concentrations on the analyte-side of the glucose membrane, which is the quantity being measured.
References
External links
Clark-type Sensors Explained
The Gas Detector Encyclopedia, Edaphic Scientific Knowledge Base
Clark Oxygen Electrode, precursor to today's modern biosensors
- broken link {{DEFAULTSORT:Clark Electrode Electrodes Gas sensors