Chronoamperometry on:
[Wikipedia]
[Google]
[Amazon]
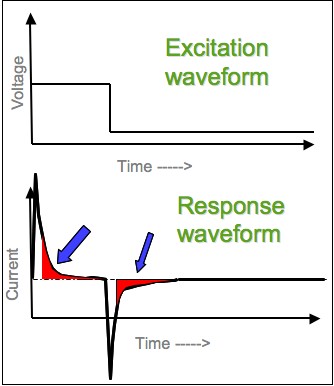 In
In  There are two types of chronoamperometry that are commonly used: controlled-potential chronoamperometry and controlled-current chronoamperometry. Before running controlled-potential chronoamperometry, cyclic voltammetries are run to determine the reduction potential of the
There are two types of chronoamperometry that are commonly used: controlled-potential chronoamperometry and controlled-current chronoamperometry. Before running controlled-potential chronoamperometry, cyclic voltammetries are run to determine the reduction potential of the
 One of the application of chronoamperometry is controlled-potential (bulk) electrolysis, which is also known as potentiostatic coulometry. During this process, a constant potential is applied to the working electrode and current is monitored over time. The analyte in one oxidation state will be oxidized or reduced to another oxidation state. The current will decrease to the base line (approaching zero) as the analyte is consumed. This process shows the total charge (in coulomb) that flows in the reaction. Total charge (n value) is calculated by integration of area under the current plot and the application of the Faraday's law.
The cell for controlled-potential (bulk) electrolysis is usually a two-compartment (divided) cell, contained a carbon rod auxiliary anode and is separated from the cathode compartment by a coarse glass frit and methyl cellulose solvent electrolyte plug. The reason for the two compartment cell is to separate cathodic and anodic reaction. The working electrode for bulk electrolysis could be a RVC disk, which has larger surface area to increase the rate of the reaction.
Controlled-potential electrolysis is normally utilized with cyclic voltammetry. Cyclic voltammetry is capable to analyse the electrochemical behavior of the analyte or the reaction. For instance, cyclic voltammetry could tell us the cathodic potential of an analyte. Since the cathodic potential of this analyte is obtained, controlled-potential electrolysis could hold this constant potential for the reaction to happen.
One of the application of chronoamperometry is controlled-potential (bulk) electrolysis, which is also known as potentiostatic coulometry. During this process, a constant potential is applied to the working electrode and current is monitored over time. The analyte in one oxidation state will be oxidized or reduced to another oxidation state. The current will decrease to the base line (approaching zero) as the analyte is consumed. This process shows the total charge (in coulomb) that flows in the reaction. Total charge (n value) is calculated by integration of area under the current plot and the application of the Faraday's law.
The cell for controlled-potential (bulk) electrolysis is usually a two-compartment (divided) cell, contained a carbon rod auxiliary anode and is separated from the cathode compartment by a coarse glass frit and methyl cellulose solvent electrolyte plug. The reason for the two compartment cell is to separate cathodic and anodic reaction. The working electrode for bulk electrolysis could be a RVC disk, which has larger surface area to increase the rate of the reaction.
Controlled-potential electrolysis is normally utilized with cyclic voltammetry. Cyclic voltammetry is capable to analyse the electrochemical behavior of the analyte or the reaction. For instance, cyclic voltammetry could tell us the cathodic potential of an analyte. Since the cathodic potential of this analyte is obtained, controlled-potential electrolysis could hold this constant potential for the reaction to happen.
 Double potential step chronoamperometry (DPSCA) is the technique whose working electrode is applied by the potential stepping forward for a certain period of time and backward for a period of time. The current is monitored and plotted with respect to time. This method starts with an induction period. In this period, several initial conditions will be applied to the electrochemical cell so that cell is able to equilibrate to those conditions. The working electrode potential will be held at the initial potential under these conditions for a specified period (i.e. usually 3 seconds). When the induction period is over, the working cells switch to another potential for a certain amount of time. After the first step is completed, the working electrode's potential is stepped back, usually to the potential prior to the forward step. The whole experiment ends with a relaxation period. Under this period, the default condition involves holding the working electrode potential of initial state for another approximate 1 seconds. When the relaxation period is over, the post experiment idle conditions will be applied to the cell so that the instrument can return to the idle state1. After plotting the current as a function of time, a chronoamperogram will occur and it can also be used to generate Cottrell plots.
Double potential step chronoamperometry (DPSCA) is the technique whose working electrode is applied by the potential stepping forward for a certain period of time and backward for a period of time. The current is monitored and plotted with respect to time. This method starts with an induction period. In this period, several initial conditions will be applied to the electrochemical cell so that cell is able to equilibrate to those conditions. The working electrode potential will be held at the initial potential under these conditions for a specified period (i.e. usually 3 seconds). When the induction period is over, the working cells switch to another potential for a certain amount of time. After the first step is completed, the working electrode's potential is stepped back, usually to the potential prior to the forward step. The whole experiment ends with a relaxation period. Under this period, the default condition involves holding the working electrode potential of initial state for another approximate 1 seconds. When the relaxation period is over, the post experiment idle conditions will be applied to the cell so that the instrument can return to the idle state1. After plotting the current as a function of time, a chronoamperogram will occur and it can also be used to generate Cottrell plots.
 The application of chronopotentiometry could be derived into two parts. As an analytical method, the range of analysis is normally in the range of 10−4 mol/L to 10−2 mol/L, and sometimes it will be as accurate as 10−5 mol/L. When the analysis is in the extreme lower range of concentration, lower current density could be used. Also, to get the accurate concentration determination, the transition time could be extended. In this area of analysis determination, chronopotentiometry is similar to
The application of chronopotentiometry could be derived into two parts. As an analytical method, the range of analysis is normally in the range of 10−4 mol/L to 10−2 mol/L, and sometimes it will be as accurate as 10−5 mol/L. When the analysis is in the extreme lower range of concentration, lower current density could be used. Also, to get the accurate concentration determination, the transition time could be extended. In this area of analysis determination, chronopotentiometry is similar to
 In
In electrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between Electric potential, electrical potential difference and identifiable chemical change. These reactions involve Electron, electrons moving via an electronic ...
, chronoamperometry is an analytical technique in which the electric potential
Electric potential (also called the ''electric field potential'', potential drop, the electrostatic potential) is defined as electric potential energy per unit of electric charge. More precisely, electric potential is the amount of work (physic ...
of the working electrode
Working may refer to:
* Work (human activity), intentional activity people perform to support themselves, others, or the community
Arts and media
* ''Working'' (musical), a 1978 musical
* ''Working'' (TV series), an American sitcom
* ''Workin ...
is stepped and the resulting current
Currents, Current or The Current may refer to:
Science and technology
* Current (fluid), the flow of a liquid or a gas
** Air current, a flow of air
** Ocean current, a current in the ocean
*** Rip current, a kind of water current
** Current (hydr ...
from faradaic processes occurring at the electrode (caused by the potential step) is monitored as a function of time. The functional relationship between current response and time is measured after applying single or double potential step to the working electrode of the electrochemical system. Limited information about the identity of the electrolyzed species can be obtained from the ratio of the peak oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
current versus the peak reduction current. However, as with all pulsed techniques, chronoamperometry generates high charging currents, which decay exponentially with time as any RC circuit
A resistor–capacitor circuit (RC circuit), or RC filter or RC network, is an electric circuit composed of resistors and capacitors. It may be driven by a voltage source, voltage or current source and these will produce different responses. A fi ...
. The Faradaic current
In electrochemistry, the faradaic current is the electric current generated by the reduction or oxidation of some chemical substance at an electrode. The net faradaic current is the algebraic sum of all the faradaic currents flowing through an i ...
- which is due to electron transfer
Electron transfer (ET) occurs when an electron relocates from an atom, ion, or molecule, to another such chemical entity. ET describes the mechanism by which electrons are transferred in redox reactions.
Electrochemical processes are ET reactio ...
events and is most often the current component of interest - decays as described in the Cottrell equation
In electrochemistry, the Cottrell equation describes the change in electric current with respect to time in a controlled potential experiment, such as chronoamperometry. Specifically it describes the current response when the potential is a step ...
. In most electrochemical cell
An electrochemical cell is a device that either generates electrical energy from chemical reactions in a so called galvanic cell, galvanic or voltaic cell, or induces chemical reactions (electrolysis) by applying external electrical energy in an ...
s, this decay is much slower than the charging decay-cells with no supporting electrolyte
An electrolyte is a substance that conducts electricity through the movement of ions, but not through the movement of electrons. This includes most soluble Salt (chemistry), salts, acids, and Base (chemistry), bases, dissolved in a polar solven ...
are notable exceptions. Most commonly a three-electrode system is used. Since the current is integrated over relatively longer time intervals, chronoamperometry gives a better signal-to-noise ratio
Signal-to-noise ratio (SNR or S/N) is a measure used in science and engineering that compares the level of a desired signal to the level of background noise. SNR is defined as the ratio of signal power to noise power, often expressed in deci ...
in comparison to other amperometric techniques.
 There are two types of chronoamperometry that are commonly used: controlled-potential chronoamperometry and controlled-current chronoamperometry. Before running controlled-potential chronoamperometry, cyclic voltammetries are run to determine the reduction potential of the
There are two types of chronoamperometry that are commonly used: controlled-potential chronoamperometry and controlled-current chronoamperometry. Before running controlled-potential chronoamperometry, cyclic voltammetries are run to determine the reduction potential of the analyte
An analyte, component (in clinical chemistry), titrand (in titrations), or chemical species is a substance or chemical constituent that is of interest in an analytical procedure. The remainder of the sample is called the matrix. The procedure ...
s. Generally, chronoamperometry uses fixed-area electrodes, which are suitable for studying electrode processes of coupled chemical reactions, especially the reaction mechanism of organic electrochemistry.
Example
Anthracene
Anthracene is a solid polycyclic aromatic hydrocarbon (PAH) of formula C14H10, consisting of three fused benzene rings. It is a component of coal tar. Anthracene is used in the production of the red dye alizarin and other dyes, as a scintil ...
in deoxygenated dimethylformamide
Dimethylformamide, DMF is an organic compound with the chemical formula . Its structure is . Commonly abbreviated as DMF (although this initialism is sometimes used for 2,5-dimethylfuran, dimethylfuran, or dimethyl fumarate), this colourless liqui ...
(DMF) will be reduced (An + e− -> An−) at the electrode surface that is at a certain negative potential
Potential generally refers to a currently unrealized ability. The term is used in a wide variety of fields, from physics to the social sciences to indicate things that are in a state where they are able to change in ways ranging from the simple r ...
. The reduction will be diffusion-limited, thereby causing the current
Currents, Current or The Current may refer to:
Science and technology
* Current (fluid), the flow of a liquid or a gas
** Air current, a flow of air
** Ocean current, a current in the ocean
*** Rip current, a kind of water current
** Current (hydr ...
to drop in time (proportional to the diffusion gradient that is formed by diffusion).
You can do this experiment several times increasing electrode potentials from low to high. (In between the experiments, the solution should be stirred.) When you measure the current i(t) at a certain fixed time point τ after applying the voltage, you will see that at a certain moment the current i(τ) does not rise anymore; you have reached the mass-transfer-limited region. This means that anthracene arrives as fast as diffusion can bring it to the electrode.
History
In 1902, F. G. Cottrell deduced the linear diffusion on a planar electrode according to the diffusion law andLaplace transform
In mathematics, the Laplace transform, named after Pierre-Simon Laplace (), is an integral transform that converts a Function (mathematics), function of a Real number, real Variable (mathematics), variable (usually t, in the ''time domain'') to a f ...
, and obtained the Cottrell equation
In electrochemistry, the Cottrell equation describes the change in electric current with respect to time in a controlled potential experiment, such as chronoamperometry. Specifically it describes the current response when the potential is a step ...
:
:
where
* is the current in amps;
* is the number of electrons;
* is the Faraday constant
In physical chemistry, the Faraday constant (symbol , sometimes stylized as ℱ) is a physical constant defined as the quotient of the total electric charge () by the amount () of elementary charge carriers in any given sample of matter: it ...
;
* is the area of the planar electrode in cm2;
* is the initial concentration of the analyte in mol/cm3;
* is the diffusion coefficient
Diffusivity, mass diffusivity or diffusion coefficient is usually written as the proportionality constant between the molar flux due to molecular diffusion and the negative value of the gradient in the concentration of the species. More accurate ...
for species in cm2/s;
* is the time in seconds.
Under controlled-diffusion circumstances, the current-time plot reflects the concentration gradient of the solution near the electrode surface. The current is directly proportional to the concentration at the electrode surface.
In 1922, Jaroslav Heyrovský
Jaroslav Heyrovský (; 20 December 1890 – 27 March 1967) was a Czech chemist and inventor who received the Nobel Prize in Chemistry in 1959 for his invention of polarography.
Life and work
Jaroslav Heyrovský was born in Prague on December 2 ...
reiterated the chronoamperometric method when he invented the polarographic method. It can use the basic circuit of the polarograph. To connect the fast recorder or oscilloscope
An oscilloscope (formerly known as an oscillograph, informally scope or O-scope) is a type of electronic test instrument that graphically displays varying voltages of one or more signals as a function of time. Their main purpose is capturing i ...
, the dropping mercury electrode
A liquid metal electrode is an electrode that uses a liquid metal, such as mercury, Galinstan, and NaK. They can be used in electrocapillarity, voltammetry, and impedance measurements.
Dropping mercury electrode
The dropping mercury electro ...
is not used, instead, the static electrodes such as suspended mercury, mercury poll or platinum
Platinum is a chemical element; it has Symbol (chemistry), symbol Pt and atomic number 78. It is a density, dense, malleable, ductility, ductile, highly unreactive, precious metal, precious, silverish-white transition metal. Its name origina ...
, gold
Gold is a chemical element; it has chemical symbol Au (from Latin ) and atomic number 79. In its pure form, it is a brightness, bright, slightly orange-yellow, dense, soft, malleable, and ductile metal. Chemically, gold is a transition metal ...
and graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable ...
are used. In addition, the solution is not stirred. In the presence of the inert electrolytes, the mass transfer process is mainly diffusion. Jarroslav Herovsky derived the chronopotentiometric method from the Cottrell equation. Chronopotentiometry is an electrochemical method that can generate a stable current that can flow between two different electrodes.
Application
Controlled-potential (bulk) electrolysis
 One of the application of chronoamperometry is controlled-potential (bulk) electrolysis, which is also known as potentiostatic coulometry. During this process, a constant potential is applied to the working electrode and current is monitored over time. The analyte in one oxidation state will be oxidized or reduced to another oxidation state. The current will decrease to the base line (approaching zero) as the analyte is consumed. This process shows the total charge (in coulomb) that flows in the reaction. Total charge (n value) is calculated by integration of area under the current plot and the application of the Faraday's law.
The cell for controlled-potential (bulk) electrolysis is usually a two-compartment (divided) cell, contained a carbon rod auxiliary anode and is separated from the cathode compartment by a coarse glass frit and methyl cellulose solvent electrolyte plug. The reason for the two compartment cell is to separate cathodic and anodic reaction. The working electrode for bulk electrolysis could be a RVC disk, which has larger surface area to increase the rate of the reaction.
Controlled-potential electrolysis is normally utilized with cyclic voltammetry. Cyclic voltammetry is capable to analyse the electrochemical behavior of the analyte or the reaction. For instance, cyclic voltammetry could tell us the cathodic potential of an analyte. Since the cathodic potential of this analyte is obtained, controlled-potential electrolysis could hold this constant potential for the reaction to happen.
One of the application of chronoamperometry is controlled-potential (bulk) electrolysis, which is also known as potentiostatic coulometry. During this process, a constant potential is applied to the working electrode and current is monitored over time. The analyte in one oxidation state will be oxidized or reduced to another oxidation state. The current will decrease to the base line (approaching zero) as the analyte is consumed. This process shows the total charge (in coulomb) that flows in the reaction. Total charge (n value) is calculated by integration of area under the current plot and the application of the Faraday's law.
The cell for controlled-potential (bulk) electrolysis is usually a two-compartment (divided) cell, contained a carbon rod auxiliary anode and is separated from the cathode compartment by a coarse glass frit and methyl cellulose solvent electrolyte plug. The reason for the two compartment cell is to separate cathodic and anodic reaction. The working electrode for bulk electrolysis could be a RVC disk, which has larger surface area to increase the rate of the reaction.
Controlled-potential electrolysis is normally utilized with cyclic voltammetry. Cyclic voltammetry is capable to analyse the electrochemical behavior of the analyte or the reaction. For instance, cyclic voltammetry could tell us the cathodic potential of an analyte. Since the cathodic potential of this analyte is obtained, controlled-potential electrolysis could hold this constant potential for the reaction to happen.
Double potential step chronoamperometry
 Double potential step chronoamperometry (DPSCA) is the technique whose working electrode is applied by the potential stepping forward for a certain period of time and backward for a period of time. The current is monitored and plotted with respect to time. This method starts with an induction period. In this period, several initial conditions will be applied to the electrochemical cell so that cell is able to equilibrate to those conditions. The working electrode potential will be held at the initial potential under these conditions for a specified period (i.e. usually 3 seconds). When the induction period is over, the working cells switch to another potential for a certain amount of time. After the first step is completed, the working electrode's potential is stepped back, usually to the potential prior to the forward step. The whole experiment ends with a relaxation period. Under this period, the default condition involves holding the working electrode potential of initial state for another approximate 1 seconds. When the relaxation period is over, the post experiment idle conditions will be applied to the cell so that the instrument can return to the idle state1. After plotting the current as a function of time, a chronoamperogram will occur and it can also be used to generate Cottrell plots.
Double potential step chronoamperometry (DPSCA) is the technique whose working electrode is applied by the potential stepping forward for a certain period of time and backward for a period of time. The current is monitored and plotted with respect to time. This method starts with an induction period. In this period, several initial conditions will be applied to the electrochemical cell so that cell is able to equilibrate to those conditions. The working electrode potential will be held at the initial potential under these conditions for a specified period (i.e. usually 3 seconds). When the induction period is over, the working cells switch to another potential for a certain amount of time. After the first step is completed, the working electrode's potential is stepped back, usually to the potential prior to the forward step. The whole experiment ends with a relaxation period. Under this period, the default condition involves holding the working electrode potential of initial state for another approximate 1 seconds. When the relaxation period is over, the post experiment idle conditions will be applied to the cell so that the instrument can return to the idle state1. After plotting the current as a function of time, a chronoamperogram will occur and it can also be used to generate Cottrell plots.
Two methods from chronoanalysis
Chronopotentiometry
 The application of chronopotentiometry could be derived into two parts. As an analytical method, the range of analysis is normally in the range of 10−4 mol/L to 10−2 mol/L, and sometimes it will be as accurate as 10−5 mol/L. When the analysis is in the extreme lower range of concentration, lower current density could be used. Also, to get the accurate concentration determination, the transition time could be extended. In this area of analysis determination, chronopotentiometry is similar to
The application of chronopotentiometry could be derived into two parts. As an analytical method, the range of analysis is normally in the range of 10−4 mol/L to 10−2 mol/L, and sometimes it will be as accurate as 10−5 mol/L. When the analysis is in the extreme lower range of concentration, lower current density could be used. Also, to get the accurate concentration determination, the transition time could be extended. In this area of analysis determination, chronopotentiometry is similar to polarography
Polarography is a type of voltammetry where the working electrode is a dropping mercury electrode (DME) or a static mercury drop electrode (SMDE), which are useful for their wide cathodic ranges and renewable surfaces. It was invented in 1922 by C ...
. Waves that are separable in polarography is also separable in chronopotentiometry.
Chronopotentiometry is an effective method to study electrode mechanism. Different electrode will have different relationship between E and t in the chronopotentiometry graph. In this situation, E is the electrode potential in voltage and t is the reaction time in seconds. By the method of studying the relationship between E and t in the chronopotentiometry graph, we can get the information of mechanisms of electrode reactions, such as the electrode reaction of hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscosity, viscous than Properties of water, water. It is used as an oxidizer, bleaching agent, and antiseptic, usua ...
and oxalic acid
Oxalic acid is an organic acid with the systematic name ethanedioic acid and chemical formula , also written as or or . It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name i ...
. The chronopotentiometry experiment could be done in a very short time period, so it is a good method to study the adsorption behavior at the electrode surface. By studying the chronopotentiometry graph of electrode after adsorption of iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
ions, it is proved that the adsorption of platinum
Platinum is a chemical element; it has Symbol (chemistry), symbol Pt and atomic number 78. It is a density, dense, malleable, ductility, ductile, highly unreactive, precious metal, precious, silverish-white transition metal. Its name origina ...
on iron ions exists. By studying the chronopotentiometry graph of platinum electrode adsorbing iodine
Iodine is a chemical element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at , and boils to a vi ...
, it is proved that the adsorption of iodine occurs in the form of iodine molecules, not iodine atoms.
Chronocoulometry
Chronocoulometry is an analytical method that has similar principle with chronoamperometry, but it monitors the relationship between charge and time instead of current and time. Chronocoulometry has the following differences with chronoamperometry: the signal increases over time instead of decreasing; the act of integration minimizes noise, resulting in a smooth hyperbolic response curve; and contributions from double-layer charging and absorbed species are easily observed.See also
*Electroanalytical methods
Electroanalytical methods are a class of techniques in analytical chemistry which study an analyte by measuring the potential (volts) and/or current (amperes) in an electrochemical cell containing the analyte. These methods can be broken down into ...
* Electrochemical skin conductance
Electrochemical skin conductance (ESC) is an objective, non-invasive and quantitative electrophysiological measure of skin conductance through the application of a pulsating direct current on the skin. It is based on reverse iontophoresis and ...
* Potentiometric titration
In analytical chemistry, potentiometric titration is a technique similar to direct titration of a redox reaction. It is a useful means of characterizing an acid. No indicator is used; instead the electric potential is measured across the analyte, ...
* Voltammetry
Voltammetry is a category of electroanalytical methods used in analytical chemistry and various industrial processes. In voltammetry, information about an analyte is obtained by measuring the current as the potential is varied. The analytical d ...
References
{{Electroanalytical Electroanalytical methods