Chlorpheniramine on:
[Wikipedia]
[Google]
[Amazon]
Chlorphenamine (CP, CPM), also known as chlorpheniramine, is an antihistamine used to treat the symptoms of allergic conditions such as allergic rhinitis (hay fever). It is taken orally (by mouth). The medication takes effect within two hours and lasts for about 4–6 hours. It is a first-generation antihistamine and works by blocking the histamine H1 receptor.
Common side effects include sleepiness, restlessness, and weakness. Other side effects may include dry mouth and wheeziness.
Chlorpheniramine was patented in 1948 and came into medical use in 1949. It is available as a generic medication and over the counter.
In 2022, it was the 291st most commonly prescribed medication in the United States, with more than 400,000 prescriptions.
Medical uses
Combination products
Chlorphenamine is often combined with phenylpropanolamine to form an allergy medication with both antihistamine and decongestant properties, although phenylpropanolamine was removed from the U.S. market per studies concluding that it increased the risk of stroke in young women. Vernate was a trade name of one such product available in the U.S. prior to the FDA ban; it was manufactured by Tutag and was among the medications prescribed toElvis Presley
Elvis Aaron Presley (January 8, 1935 – August 16, 1977) was an American singer and actor. Referred to as the "King of Rock and Roll", he is regarded as Cultural impact of Elvis Presley, one of the most significant cultural figures of the ...
.
In the drug Coricidin, chlorphenamine is combined with the cough suppressant dextromethorphan
Dextromethorphan, sold under the brand name Robitussin among others, is a cough suppressant used in many cough and Common cold, cold medicines. In 2022, the US Food and Drug Administration (FDA) approved the combination dextromethorphan/bupropi ...
. In the drug Cêgripe, chlorphenamine is combined with the analgesic paracetamol (also known as acetaminophen, sold as ''Tylenol'').
Side effects
The adverse effects include drowsiness, dizziness, confusion, constipation, anxiety, nausea, blurred vision, restlessness, decreased coordination, dry mouth, shallow breathing, hallucinations, irritability, problems with memory or concentration, tinnitus and trouble urinating. Chlorphenamine produces less sedation than other first-generation antihistamines. A large study on people 65 years old or older linked the development of Alzheimer's disease and other forms of dementia to the "higher cumulative" use of chlorphenamine and other first-generation antihistamines, due to theiranticholinergic
Anticholinergics (anticholinergic agents) are substances that block the action of the acetylcholine (ACh) neurotransmitter at synapses in the central nervous system, central and peripheral nervous system.
These agents inhibit the parasympatheti ...
properties. Chlorphenamine is rated as a "high burden" anticholinergic by experts on a semi-subjective scale. This is inconsistent with the ''in vitro'' experiments showing low affinity to muscarinic acetylcholine receptors (see below).
Pharmacology
Pharmacodynamics
Chlorphenamine acts primarily as a potent H1 antihistamine. It is specifically a potent inverse agonist of the histamine H1 receptor. The drug is also commonly described as possessing weakanticholinergic
Anticholinergics (anticholinergic agents) are substances that block the action of the acetylcholine (ACh) neurotransmitter at synapses in the central nervous system, central and peripheral nervous system.
These agents inhibit the parasympatheti ...
activity by acting as an antagonist
An antagonist is a character in a story who is presented as the main enemy or rival of the protagonist and is often depicted as a villain.muscarinic acetylcholine receptors. The dextrorotatory stereoisomer, dexchlorpheniramine, has been reported to possess Kd values of 15 nM for the H1 receptor and 1,300 nM for the muscarinic acetylcholine receptors in human brain tissue. The smaller the Kd value, the greater the binding affinity of the ligand for its target.
In addition to acting as an inverse agonist at the H1 receptor, chlorphenamine has been found to act as a serotonin reuptake inhibitor (Kd = 15.2 nM for the serotonin transporter). It has only weak affinity for the norepinephrine and dopamine transporters (Kd = 1,440 nM and 1,060 nM, respectively).
A study found that dexchlorphenamine had Ki values for the human cloned H1 receptor of 2.67 to 4.81 nM while levchlorphenamine had Ki values of 211 to 361 nM for this receptor, indicating that dexchlorphenamine is the active enantiomer. Another study found that dexchlorphenamine had a Ki value of 20 to 30 μM for the muscarinic acetylcholine receptor using rat brain tissue while levchlorphenamine had a Ki value of 40 to 50 μM for this receptor, indicating that both enantiomers have very low affinity for it.
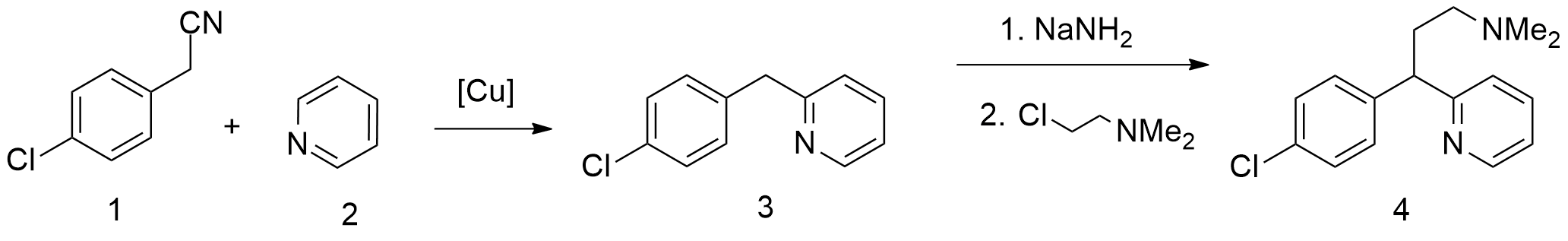
 A second method boom starts from pyridine, which undergoes alkylation by 4-chlorophenylacetonitrile, giving 2-(4-chlorobenzyl)pyridine. Alkylating this with 2-dimethylaminoethylchloride in the presence of sodium amide gives chlorphenamine.
A second method boom starts from pyridine, which undergoes alkylation by 4-chlorophenylacetonitrile, giving 2-(4-chlorobenzyl)pyridine. Alkylating this with 2-dimethylaminoethylchloride in the presence of sodium amide gives chlorphenamine.
Pharmacokinetics
The elimination half-life of chlorphenamine has variously ranged between 13.9 and 43.4 hours in adults following a single dose in clinical studies.Chemistry
Chlorphenamine is an alkylamine and is a part of a series of antihistamines including pheniramine (Naphcon) and its halogenated derivatives including fluorpheniramine, dexchlorphenamine (Polaramine), brompheniramine (Dimetapp), dexbrompheniramine (Drixoral), deschlorpheniramine, and iodopheniramine. The halogenated alkylamine antihistamines all exhibit optical isomerism, and chlorphenamine in the indicated products is racemic chlorphenamine maleate, whereas dexchlorphenamine is the dextrorotary stereoisomer.Synthesis
There are several patented methods for the synthesis of chlorphenamine. In one example, 4-chlorophenylacetonitrile is reacted with 2-chloropyridine in the presence of sodium amide to form 4-chlorophenyl(2-pyridyl)acetonitrile. Alkylating this with 2-dimethylaminoethylchloride in the presence of sodium amide gives γ-(4-chlorphenyl)-γ-cyano-''N'',''N''-dimethyl-2-pyridinepropanamine, the hydrolysis and decarboxylation of which lead to chlorphenamine. A second method boom starts from pyridine, which undergoes alkylation by 4-chlorophenylacetonitrile, giving 2-(4-chlorobenzyl)pyridine. Alkylating this with 2-dimethylaminoethylchloride in the presence of sodium amide gives chlorphenamine.
A second method boom starts from pyridine, which undergoes alkylation by 4-chlorophenylacetonitrile, giving 2-(4-chlorobenzyl)pyridine. Alkylating this with 2-dimethylaminoethylchloride in the presence of sodium amide gives chlorphenamine.

Society and culture
Names
''Chlorphenamine'' is the while ''chlorpheniramine'' is the and former . Brand names include Chlor-Trimeton, Demazin, Allerest 12 Hour, Piriton, Chlorphen-12, Tylenol Cold/Allergy, and numerous others according to country.References
{{Portal bar, Medicine Antidepressants Anxiolytics 4-Chlorophenyl compounds CYP2D6 inhibitors H1 receptor antagonists Local anesthetics Muscarinic antagonists 2-Pyridyl compounds Serotonin reuptake inhibitors Sigma receptor modulators Sodium channel blockers Dimethylamino compounds