Chemical Structure Determination on:
[Wikipedia]
[Google]
[Amazon]
 A chemical structure determination includes a
A chemical structure determination includes a
MOGADOC
A data base for experimental structures determined in the gas phase *
 A chemical structure determination includes a
A chemical structure determination includes a chemist
A chemist (from Greek ''chēm(ía)'' alchemy; replacing ''chymist'' from Medieval Latin ''alchemist'') is a scientist trained in the study of chemistry. Chemists study the composition of matter and its properties. Chemists carefully describe th ...
's specifying the molecular geometry and, when feasible and necessary, the electronic structure of the target molecule or other solid. Molecular geometry refers to the spatial arrangement of atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, and ...
s in a molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
and the chemical bonds that hold the atoms together, and can be represented using structural formula
The structural formula of a chemical compound is a graphic representation of the molecular structure (determined by structural chemistry methods), showing how the atoms are possibly arranged in the real three-dimensional space. The chemical bondi ...
e and by molecular models; complete electronic structure descriptions include specifying the occupation of a molecule's molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding ...
s. Structure determination can be applied to a range of targets from very simple molecules (e.g., diatomic
Diatomic molecules () are molecules composed of only two atoms, of the same or different chemical elements. If a diatomic molecule consists of two atoms of the same element, such as hydrogen () or oxygen (), then it is said to be homonuclear. Ot ...
oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
or nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
), to very complex ones (e.g., such as protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respo ...
or DNA).
Background
Theories of chemical structure were first developed by August Kekulé,Archibald Scott Couper
Archibald Scott Couper (; 31 March 1831 – 11 March 1892) was a Scottish chemist who proposed an early theory of chemical structure and bonding. He developed the concepts of tetravalent carbon atoms linking together to form large molecules ...
, and Aleksandr Butlerov, among others, from about 1858. These theories were first to state that chemical compounds are not a random cluster of atoms and functional groups, but rather had a definite order defined by the valency of the atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, and ...
s composing the molecule, giving the molecules a three dimensional structure that could be determined or solved.
Concerning chemical structure one has to distinguish between pure connectivity of the atoms within a molecule (chemical constitution), a description of a three-dimensional arrangement ( molecular configuration, includes e.g. information on chirality
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from ...
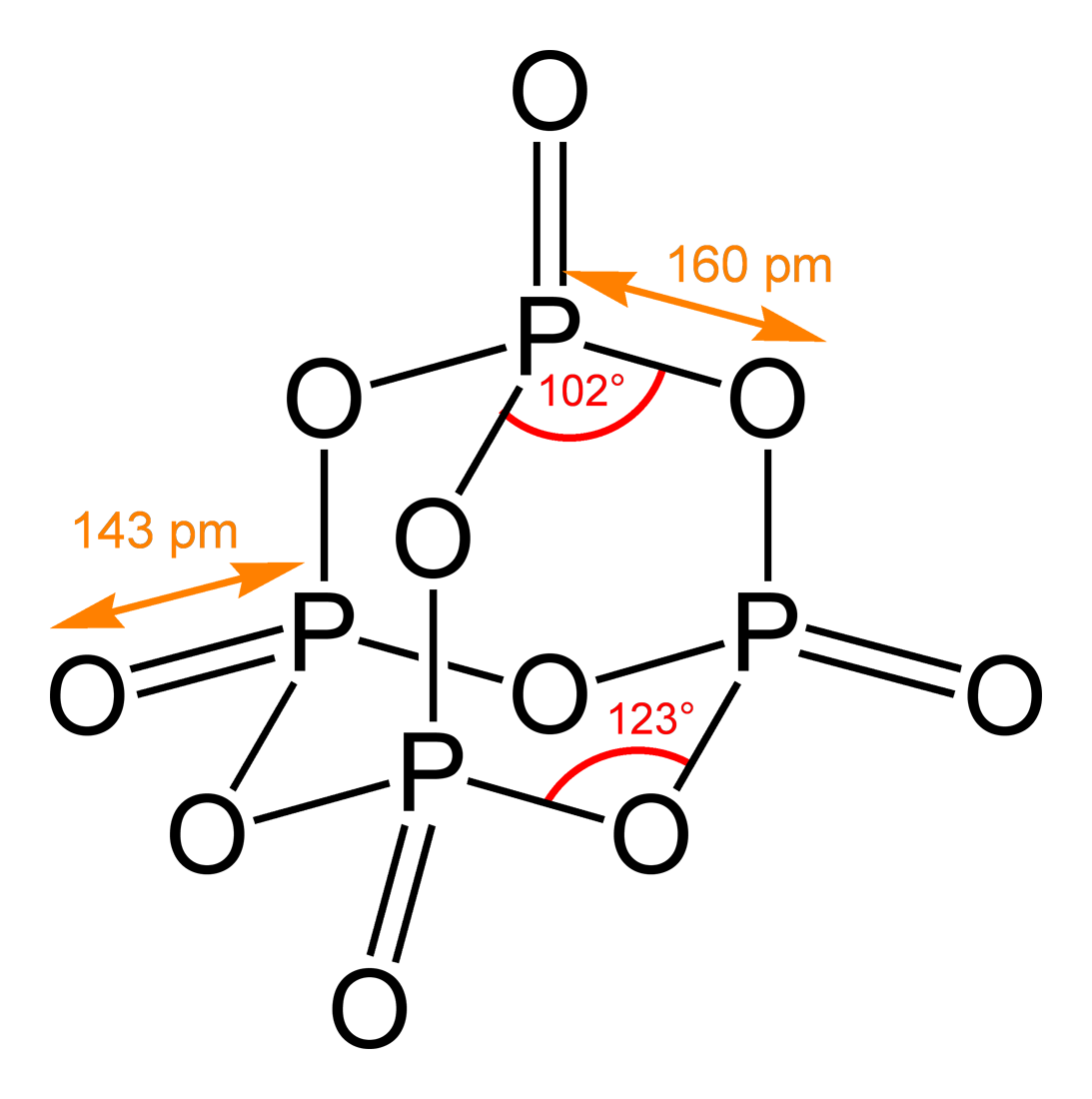
) and the precise determination of bond lengths, angles and torsion angles, i.e. a full representation of the (relative) atomic coordinates.
In determining structures of chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
s, one generally aims to obtain, first and minimally, the pattern and degree of bonding between all atoms in the molecule; when possible, one seeks the three dimensional spatial coordinates of the atoms in the molecule (or other solid).
The methods by which one can elucidate the structure of a molecule include:
* concerning only connectivity of the atoms: spectroscopies such as nuclear magnetic resonance (proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
and carbon-13 NMR Carbon-13 (C13) nuclear magnetic resonance (most commonly known as carbon-13 NMR spectroscopy or 13C NMR spectroscopy or sometimes simply referred to as carbon NMR) is the application of nuclear magnetic resonance (NMR) spectroscopy to carbon. It is ...
), various methods of mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is use ...
(to give overall molecular mass, as well as fragment masses).Techniques such as absorption spectroscopy and the vibrational spectroscopies, infrared
Infrared (IR), sometimes called infrared light, is electromagnetic radiation (EMR) with wavelengths longer than those of visible light. It is therefore invisible to the human eye. IR is generally understood to encompass wavelengths from around ...
and Raman, provide, respectively, important supporting information about the numbers and adjacencies of multiple bonds, and about the types of functional groups (whose internal bonding gives vibrational signatures); further inferential studies that give insight into the contributing electronic structure of molecules include cyclic voltammetry
Cyclic voltammetry (CV) is a type of potentiodynamic electrochemical measurement. In a cyclic voltammetry experiment, the working electrode potential is ramped linearly versus time. Unlike in linear sweep voltammetry, after the set potential is re ...
and X-ray photoelectron spectroscopy.
* concerning precise metric three-dimensional information: can be obtained for gases by gas electron diffraction
Gas electron diffraction (GED) is one of the applications of electron diffraction techniques. The target of this method is the determination of the structure of gaseous molecules, i.e., the geometrical arrangement of the atoms from which a molec ...
and microwave (rotational) spectroscopy (and other rotationally resolved spectroscopy) and for the crystalline solid state by X-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
or neutron diffraction. These technique can produce three-dimensional models at atomic-scale resolution
Resolution(s) may refer to:
Common meanings
* Resolution (debate), the statement which is debated in policy debate
* Resolution (law), a written motion adopted by a deliberative body
* New Year's resolution, a commitment that an individual mak ...
, typically to a precision of 0.001 Å for distances and 0.1° for angles (in unusual cases even better).
Additional sources of information are: When a molecule has an unpaired electron spin in a functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest ...
of its structure, ENDOR Endor or Ein Dor may refer to:
Places
* Endor (village), from the Hebrew Bible, a Canaanite village where the Witch of Endor lived
* Indur, a Palestinian village depopulated during the 1948 Arab-Israeli war
* Ein Dor, a Kibbutz in modern Israel
Fi ...
and electron-spin resonance spectroscopes may also be performed. These latter techniques become all the more important when the molecules contain metal atoms, and when the crystals required by crystallography or the specific atom types that are required by NMR are unavailable to exploit in the structure determination. Finally, more specialized methods such as electron microscopy
An electron microscope is a microscope that uses a beam of accelerated electrons as a source of illumination. As the wavelength of an electron can be up to 100,000 times shorter than that of visible light photons, electron microscopes have a hi ...
are also applicable in some cases.
See also
*Structural chemistry
Structural chemistry is a part of chemistry and deals with spatial structures of molecules (in the gaseous, liquid or solid state) and solids (with extended structures that cannot be subdivided into molecules).
The main tasks are:
* The formulat ...
* Chemical structure diagram
The structural formula of a chemical compound is a graphic representation of the molecular structure (determined by structural chemistry methods), showing how the atoms are possibly arranged in the real three-dimensional space. The chemical bond ...
* Crystallographic database
MOGADOC
A data base for experimental structures determined in the gas phase *
Pauli exclusion principle
In quantum mechanics, the Pauli exclusion principle states that two or more identical particles with half-integer spins (i.e. fermions) cannot occupy the same quantum state within a quantum system simultaneously. This principle was formulated ...
* Chemical graph generator
A chemical graph generator is a software package to generate computer representations of chemical structures adhering to certain boundary conditions. The development of such software packages is a research topic of cheminformatics. Chemical graph g ...
References
Further reading
* * {{Cite book , last1=Ward , first1=S. C. , url=https://journals.iucr.org/b/issues/2016/02/00/bm5086/index.html#BB59 , title=The Cambridge Structural Database , last2=Lightfoot , first2=M. P. , last3=Bruno , first3=I. J. , last4=Groom , first4=C. R. , date=2016-04-01 , work=Acta Crystallographica Section B , volume=72 , pages=171–179 , language=en , doi=10.1107/S2052520616003954 , issn=2052-5206 , pmc=4822653 , pmid=27048719 , doi-access=free , issue=2 Analytical chemistry