Chemical looping combustion on:
[Wikipedia]
[Google]
[Amazon]
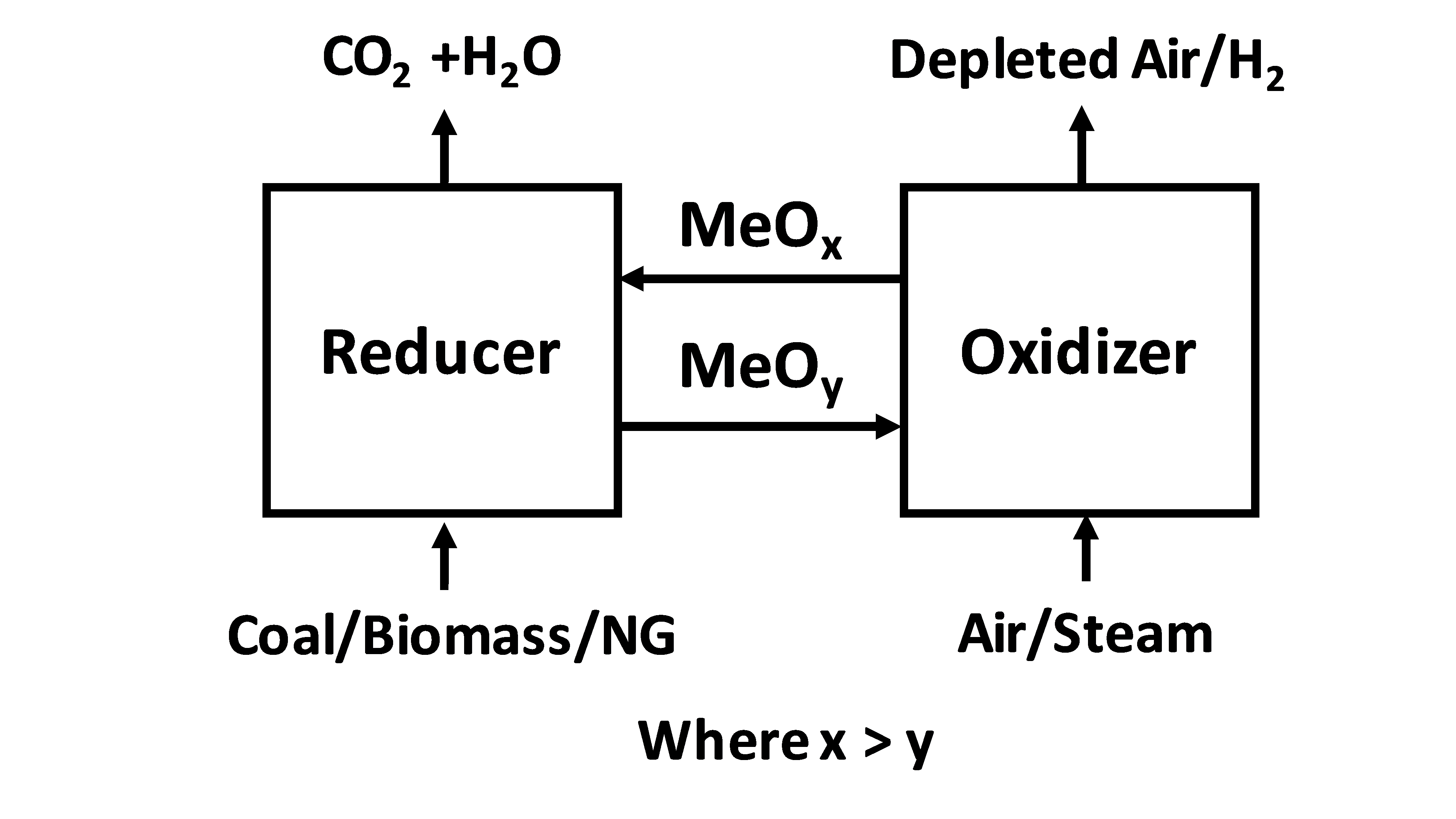
 Chemical looping combustion (CLC) is a technological process typically employing a dual
Chemical looping combustion (CLC) is a technological process typically employing a dual
chemical-looping.at
* * * * {{cite web , url=http://www.encapco2.org/sp4.htm , url-status=dead , archive-url=https://web.archive.org/web/20080421171127/http://www.encapco2.org/sp4.htm , archive-date=2008-04-21 , title=SP4: Chemical Looping Combustion , website=ENCAP *
Carbon capture and chemical looping technology - an update on progress
. Webinar recording, Carl Bozzuto and the Global CCS Institute, 11 July 2012. Combustion looping combustion

 Chemical looping combustion (CLC) is a technological process typically employing a dual
Chemical looping combustion (CLC) is a technological process typically employing a dual fluidized bed
A fluidized bed is a physical phenomenon that occurs when a solid particulate substance (usually present in a holding vessel) is under the right conditions so that it behaves like a fluid. The usual way to achieve a fluidized bed is to pump press ...
system. CLC operated with an interconnected moving bed with a fluidized bed system, has also been employed as a technology process. In CLC, a metal oxide is employed as a bed material providing the oxygen for combustion in the fuel reactor. The reduced metal is then transferred to the second bed ( air reactor) and re-oxidized before being reintroduced back to the fuel reactor completing the loop. Fig 1 shows a simplified diagram of the CLC process. Fig 2 shows an example of a dual fluidized bed circulating reactor system and a moving bed-fluidized bed circulating reactor system.
Isolation of the fuel from air
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosph ...
simplifies the number of chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
s in combustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion ...
. Employing oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
without nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
and the trace gases found in air eliminates the primary source for the formation of nitrogen oxide
Nitrogen oxide may refer to a binary compound of oxygen and nitrogen, or a mixture of such compounds:
Charge-neutral
*Nitric oxide (NO), nitrogen(II) oxide, or nitrogen monoxide
* Nitrogen dioxide (), nitrogen(IV) oxide
* Nitrogen trioxide (), o ...
(), produces a flue gas composed primarily of carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
and water vapor
Water vapor, water vapour, or aqueous vapor is the gaseous phase of Properties of water, water. It is one Phase (matter), state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from th ...
; other trace pollutants
A pollutant or novel entity is a substance or energy introduced into the environment that has undesired effect, or adversely affects the usefulness of a resource. These can be both naturally forming (i.e. minerals or extracted compounds like oi ...
depend on the fuel
A fuel is any material that can be made to react with other substances so that it releases energy as thermal energy or to be used for work (physics), work. The concept was originally applied solely to those materials capable of releasing chem ...
selected.
Description
Chemical looping combustion (CLC) uses two or more reactions to perform the oxidation of hydrocarbon-based fuels. In its simplest form, an oxygen-carrying species (normally a metal) is first oxidized in the air forming an oxide. This oxide is then reduced using a hydrocarbon as a reducer in a second reaction. As an example, aniron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
based system burning pure carbon would involve the two redox
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is t ...
reactions:
If () and () are added together, the reaction set reduces to straight carbon oxidation i.e.:
CLC was first studied as a way to produce from fossil fuels, using two interconnected fluidized beds. Later it was proposed as a system for increasing power station efficiency. The gain in efficiency is possible due to the enhanced reversibility of the two redox reactions; in traditional single stage combustion, the release of a fuel's energy occurs in a highly irreversible manner - departing considerably from equilibrium. In CLC, if an appropriate oxygen carrier is chosen, both redox reactions can be made to occur almost reversibly and at relatively low temperatures. Theoretically, this allows a power station using CLC to approach the ideal work output for an internal combustion engine without exposing components to excessive working temperatures.
Thermodynamics
Fig 3 illustrates the energy exchanges in a CLC system graphically and shows a Sankey diagram of the energy fluxes occurring in a reversible CLC based engine. Studying Fig 1, aheat engine
A heat engine is a system that transfers thermal energy to do mechanical or electrical work. While originally conceived in the context of mechanical energy, the concept of the heat engine has been applied to various other kinds of energy, pa ...
is arranged to receive heat at high temperatures from the exothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity (e ...
oxidation reaction. After converting part of this energy to work, the heat engine rejects the remaining energy as heat. Almost all of this heat rejection can be absorbed by the endothermic
An endothermic process is a chemical or physical process that absorbs heat from its surroundings. In terms of thermodynamics, it is a thermodynamic process with an increase in the enthalpy (or internal energy ) of the system.Oxtoby, D. W; Gillis, ...
reduction reaction occurring in the reducer. This arrangement requires the redox reactions to be exothermic and endothermic respectively, but this is normally the case for most metals. Some additional heat exchange with the environment is required to satisfy the second law; theoretically, for a reversible process, the heat exchange is related to the standard state entropy change, ΔSo, of the primary hydrocarbon oxidation reaction as follows:
:Qo = ToΔSo
However, for most hydrocarbons, ΔSo is a small value and, as a result, an engine of high overall efficiency is theoretically possible.
CO2 capture
Although proposed as a means of increasing efficiency, in recent years, interest has been shown in CLC as a carbon capture technique. Carbon capture is facilitated by CLC because the two redox reactions generate two intrinsically separated flue gas streams: a stream from the air reactor, consisting of atmospheric and residual , but sensibly free of ; and a stream from the fuel reactor predominately containing and with very little diluent nitrogen. The air reactor flue gas can be discharged to the atmosphere causing minimal pollution. The reducer exit gas contains almost all of the generated by the system and CLC therefore can be said to exhibit 'inherent carbon capture', as water vapor can easily be removed from the second flue gas via condensation, leading to a stream of almost pure . This gives CLC clear benefits when compared with competing carbon capture technologies, as the latter generally involve a significant energy penalty associated with either post combustion scrubbing systems or the work input required for air separation plants. This has led to CLC being proposed as an energy efficient carbon capture technology, able to capture nearly all of the CO2, for example, from a Coal Direct Chemical Looping (CDCL) plant. A continuous 200-hour demonstration results of a 25 kWth CDCL sub-pilot unit indicated nearly 100% coal conversion to CO2 with no carbon carryover to the air reactor.Technology development
First operation of chemical-looping combustion with gaseous fuels was demonstrated in 2003, and later with solid fuels in 2006. Total operational experience in 49 pilots of 0.3 to 3 MW is more than 11,000 h. Oxygen carrier materials used in operation include monometallic oxides of nickel, copper, manganese and iron, as well as various combined oxides including manganese oxides.combined with calcium, iron and silica. Also natural ores have been in use, especially for solid fuels, including iron ores, manganese ores and ilmenite.Cost and energy penalty
A detailed technology assessment of chemical-looping combustion of solid fuel, i.e. coal, for a 1000 MWth power plant shows that the added CLC reactor costs as compared to a normal circulating fluidized bed boiler are small, because of the similarities of the technologies. Major costs are instead CO2 compression, needed in all CO2 capture technologies, and oxygen production. Molecular oxygen production may also be needed in certain CLC configuration for polishing the product gas from the fuel reactor. In all the added costs were estimated to 20 €/tonne of CO2 whereas the energy penalty was 4%.Variants and related technologies
A variant of CLC is Chemical-Looping Combustion with Oxygen Uncoupling (CLOU) where an oxygen carrier is used that releases gas-phase oxygen in the fuel reactor, e.g. CuO/O. This is helpful for achieving high gas conversion, and especially when using solid fuels, where slow steam gasification of char can be avoided. CLOU operation with solid fuels shows high performance Chemical Looping can also be used to produce hydrogen in Chemical-Looping Reforming (CLR) processes. In one configuration of the CLR process, hydrogen is produced from coal and/or natural gas using a moving bed fuel reactor integrated with a steam reactor and a fluidized bed air reactor. This configuration of CLR can produce greater than 99% purity H2 without the need for CO2 separation. Another CLR process is based on conventional steam methane reforming (SMR), with chemical-looping combustion replacing the reformer furnace. Thus, the heat for the reforming is transferred in fluidized-bed heat exchangers (FBHEs) connected to the air reactor and the fuel used is the off-gas from the reforming plus additional methane. This configuration can give higher energy efficiency than conventional SMR, because the furnace temperature can be reduced. Consequently, a negative CO2 capture cost could be possible. Lower temperature and more efficient heat transfer in FBHEs may also allow for thinner and shorter reformer tubes and reduce need for catalyst. Comprehensive overviews of the field are given in recent reviews on chemical looping technologies. In summary, CLC can achieve both an increase in power station efficiency simultaneously with low energy penalty carbon capture. Challenges with CLC include the operation of dual fluidized bed (maintaining carrier fluidization while avoiding crushing and attrition), and maintaining carrier stability over many cycles.See also
*Chemical looping reforming and gasification Chemical looping reforming (CLR) and gasification (CLG) are the operations that involve the use of gaseous carbonaceous feedstock and solid carbonaceous feedstock, respectively, in their conversion to syngas in the chemical looping scheme. The typic ...
* Combustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion ...
* Oxy-fuel combustion
Oxy-fuel combustion is the process of burning a fuel using pure oxygen, or a mixture of oxygen and recirculated flue gas, instead of air. Since the nitrogen component of air is not heated, fuel consumption is reduced, and higher flame temperatur ...
* Oxidizing agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ''electron donor''). In ot ...
* Redox
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is t ...
(reduction/oxidation reaction)
* Carbon capture and storage
Carbon capture and storage (CCS) is a process by which carbon dioxide (CO2) from industrial installations is separated before it is released into the atmosphere, then transported to a long-term storage location.IPCC, 2021Annex VII: Glossary at ...
* Lane hydrogen producer
References
External links
*chemical-looping.at
* * * * {{cite web , url=http://www.encapco2.org/sp4.htm , url-status=dead , archive-url=https://web.archive.org/web/20080421171127/http://www.encapco2.org/sp4.htm , archive-date=2008-04-21 , title=SP4: Chemical Looping Combustion , website=ENCAP *
Carbon capture and chemical looping technology - an update on progress
. Webinar recording, Carl Bozzuto and the Global CCS Institute, 11 July 2012. Combustion looping combustion