Chemical Force Microscopy on:
[Wikipedia]
[Google]
[Amazon]
 In
In
 This is the simpler mode of CFM operation where a functionalized tip is brought in contact with the surface and is pulled to observe the force at which separation occurs, (see Figure 2). The Johnson-Kendall-Roberts (JKR) theory of adhesion mechanics predicts this value as
(1)
where with being the radius of the tip, and being various surface energies between the tip, sample, and the medium each is in (liquids discussed below). is usually obtained from SEM and and from contact angle measurements on substrates with the given moieties. When the same functional groups are used, and which results in Doing this twice with two different moieties (e.g. and ) gives values of and , both of which can be used together in the same experiment to determine . Therefore, can be calculated for any combination of functionalities for comparison to CFM determined values.
For similarly functionalized tip and surface, at tip detachment JKR theory also predicts a contact radius of
(2)
with an “effective”
This is the simpler mode of CFM operation where a functionalized tip is brought in contact with the surface and is pulled to observe the force at which separation occurs, (see Figure 2). The Johnson-Kendall-Roberts (JKR) theory of adhesion mechanics predicts this value as
(1)
where with being the radius of the tip, and being various surface energies between the tip, sample, and the medium each is in (liquids discussed below). is usually obtained from SEM and and from contact angle measurements on substrates with the given moieties. When the same functional groups are used, and which results in Doing this twice with two different moieties (e.g. and ) gives values of and , both of which can be used together in the same experiment to determine . Therefore, can be calculated for any combination of functionalities for comparison to CFM determined values.
For similarly functionalized tip and surface, at tip detachment JKR theory also predicts a contact radius of
(2)
with an “effective” 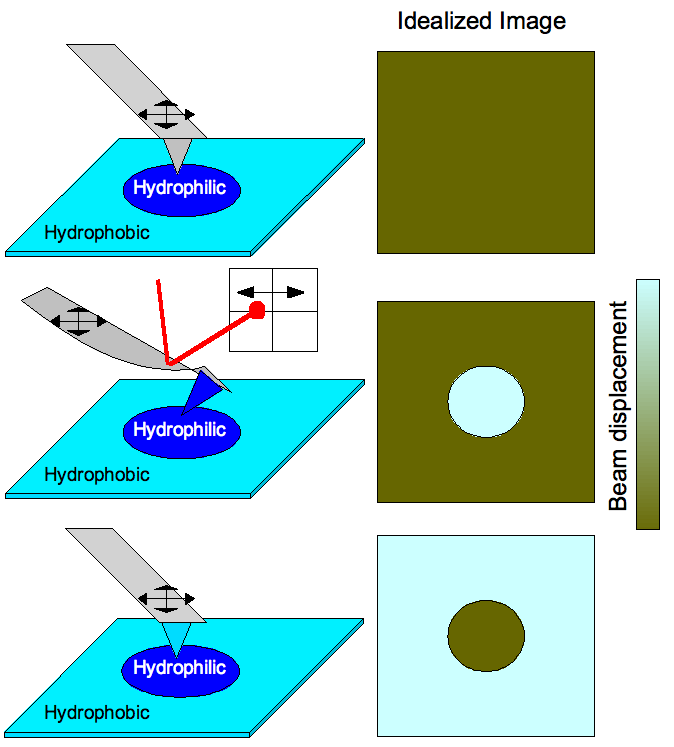 A quick note to mention is the work corresponding to the hysteresis in the force profile (Figure 2) does not correlate to the bond energy. The work done in retracting the tip is approximated due to the linear behavior of deformation with being the force and being the displacement immediately before release. Using the results of Frisbie et al., normalized to the estimated 50 functional groups in contact, the work values are estimated as 39 eV, 0.25 eV, and 4.3 eV for , , and interactions, respectively. Roughly, intermolecular bond energies can be calculated by: being the boiling point. According to this, for
A quick note to mention is the work corresponding to the hysteresis in the force profile (Figure 2) does not correlate to the bond energy. The work done in retracting the tip is approximated due to the linear behavior of deformation with being the force and being the displacement immediately before release. Using the results of Frisbie et al., normalized to the estimated 50 functional groups in contact, the work values are estimated as 39 eV, 0.25 eV, and 4.3 eV for , , and interactions, respectively. Roughly, intermolecular bond energies can be calculated by: being the boiling point. According to this, for
 Frictional force response to the amount of perpendicular load applied by the tip on to the substrate is shown in Figure 4. Increasing tip-substrate interactions produce a steeper slope, as one would expect. Of experimental importance is the fact that contrast between different functionalities on the surface may be enhanced with an application of greater perpendicular force. Of course, this comes at the cost of potential damage to the substrate.
Frictional force response to the amount of perpendicular load applied by the tip on to the substrate is shown in Figure 4. Increasing tip-substrate interactions produce a steeper slope, as one would expect. Of experimental importance is the fact that contrast between different functionalities on the surface may be enhanced with an application of greater perpendicular force. Of course, this comes at the cost of potential damage to the substrate.
 A biological implementation of CFM at the nanoscale level is the unfolding of proteins with functionalized tip and surface (see Figure 5). Due to the increased contact area, the tip and the surface act as anchors holding protein bundles while they separate. As uncoiling ensues, the force required jumps, indicating various stages of uncoiling: (1) separation into bundles, (2) bundle separation into domains of crystalline protein held together by van der Waals forces, and (3) linearization of the protein upon overcoming the secondary bonding. Information on the internal structure of these complex proteins, as well as a better understanding of constituent interactions are provided with this method.
A second consideration is one that takes advantage of unique nanoscale materials properties. The high
A biological implementation of CFM at the nanoscale level is the unfolding of proteins with functionalized tip and surface (see Figure 5). Due to the increased contact area, the tip and the surface act as anchors holding protein bundles while they separate. As uncoiling ensues, the force required jumps, indicating various stages of uncoiling: (1) separation into bundles, (2) bundle separation into domains of crystalline protein held together by van der Waals forces, and (3) linearization of the protein upon overcoming the secondary bonding. Information on the internal structure of these complex proteins, as well as a better understanding of constituent interactions are provided with this method.
A second consideration is one that takes advantage of unique nanoscale materials properties. The high
 In
In materials science
Materials science is an interdisciplinary field of researching and discovering materials. Materials engineering is an engineering field of finding uses for materials in other fields and industries.
The intellectual origins of materials sci ...
, chemical force microscopy (CFM) is a variation of atomic force microscopy
Atomic force microscopy (AFM) or scanning force microscopy (SFM) is a very-high-resolution type of scanning probe microscopy (SPM), with demonstrated resolution on the order of fractions of a nanometer, more than 1000 times better than the opti ...
(AFM) which has become a versatile tool for characterization of materials surface
A surface, as the term is most generally used, is the outermost or uppermost layer of a physical object or space. It is the portion or region of the object that can first be perceived by an observer using the senses of sight and touch, and is ...
s. With AFM, structural morphology is probed using simple tapping or contact modes that utilize van der Waals interactions
A van is a type of road vehicle used for transporting goods or people. There is some variation in the scope of the word across the different English-speaking countries. The smallest vans, microvans, are used for transporting either goods or p ...
between tip and sample to maintain a constant probe deflection amplitude (constant force mode) or maintain height while measuring tip deflection (constant height mode). CFM, on the other hand, uses chemical interactions between functionalized probe tip and sample. Choice chemistry is typically gold-coated tip and surface with thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl grou ...
s attached, R being the functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
s of interest. CFM enables the ability to determine the chemical nature of surfaces, irrespective of their specific morphology, and facilitates studies of basic chemical bonding enthalpy and surface energy
In surface science, surface energy (also interfacial free energy or surface free energy) quantifies the disruption of intermolecular bonds that occurs when a surface is created. In solid-state physics, surfaces must be intrinsically less energe ...
. Typically, CFM is limited by thermal vibration
The term "thermal energy" is often used ambiguously in physics and engineering. It can denote several different physical concepts, including:
* Internal energy: The energy contained within a body of matter or radiation, excluding the potential en ...
s within the cantilever
A cantilever is a rigid structural element that extends horizontally and is unsupported at one end. Typically it extends from a flat vertical surface such as a wall, to which it must be firmly attached. Like other structural elements, a cantilev ...
holding the probe. This limits force measurement resolution to ~1 pN, which is still very suitable considering weak interactions are ~20 pN per pair. Hydrophobicity
In chemistry, hydrophobicity is the chemical property of a molecule (called a hydrophobe) that is seemingly intermolecular force, repelled from a mass of water. In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to b ...
is used as the primary example throughout this consideration of CFM, but certainly any type of bonding can be probed with this method.
Pioneering work
CFM has been primarily developed byCharles Lieber
Charles M. Lieber (born 1959) is an American chemist, inventor, nanotechnologist, and writer. In 2011, Lieber was named the leading chemist in the world for the decade 2000–2010 by Thomson Reuters, based on the impact of his scientific publica ...
at Harvard University in 1994. The method was demonstrated using hydrophobicity where polar molecules (e.g. COOH) tend to have the strongest binding to each other, followed by nonpolar (e.g. CH3-CH3) bonding, and a combination being the weakest. Probe tips are functionalized and substrates patterned with these molecules. All combinations of functionalization were tested, both by tip contact and removal as well as spatial mapping of substrates patterned with both moieties and observing the complementarity in image contrast. Both of these methods are discussed below. The AFM instrument used is similar to the one in Figure 1.
Force of adhesion (tensile testing)
 This is the simpler mode of CFM operation where a functionalized tip is brought in contact with the surface and is pulled to observe the force at which separation occurs, (see Figure 2). The Johnson-Kendall-Roberts (JKR) theory of adhesion mechanics predicts this value as
(1)
where with being the radius of the tip, and being various surface energies between the tip, sample, and the medium each is in (liquids discussed below). is usually obtained from SEM and and from contact angle measurements on substrates with the given moieties. When the same functional groups are used, and which results in Doing this twice with two different moieties (e.g. and ) gives values of and , both of which can be used together in the same experiment to determine . Therefore, can be calculated for any combination of functionalities for comparison to CFM determined values.
For similarly functionalized tip and surface, at tip detachment JKR theory also predicts a contact radius of
(2)
with an “effective”
This is the simpler mode of CFM operation where a functionalized tip is brought in contact with the surface and is pulled to observe the force at which separation occurs, (see Figure 2). The Johnson-Kendall-Roberts (JKR) theory of adhesion mechanics predicts this value as
(1)
where with being the radius of the tip, and being various surface energies between the tip, sample, and the medium each is in (liquids discussed below). is usually obtained from SEM and and from contact angle measurements on substrates with the given moieties. When the same functional groups are used, and which results in Doing this twice with two different moieties (e.g. and ) gives values of and , both of which can be used together in the same experiment to determine . Therefore, can be calculated for any combination of functionalities for comparison to CFM determined values.
For similarly functionalized tip and surface, at tip detachment JKR theory also predicts a contact radius of
(2)
with an “effective” Young's modulus
Young's modulus (or the Young modulus) is a mechanical property of solid materials that measures the tensile or compressive stiffness when the force is applied lengthwise. It is the modulus of elasticity for tension or axial compression. Youn ...
of the tip derived from the actual value and the Poisson ratio
In materials science and solid mechanics, Poisson's ratio (symbol: (Nu (letter), nu)) is a measure of the Poisson effect, the Deformation (engineering), deformation (expansion or contraction) of a material in directions perpendicular to the spec ...
. If one knows the effective area of a single functional group, (e.g. from quantum chemistry simulations), the total number of ligands participating in tension can be estimated as As stated earlier, the force resolution of CFM does allow one to probe individual bonds of even the weakest variety, but tip curvature typically prevents this. Using Eq 2, a radius of curvature has been determined as the requirement to conduct tensile testing of individual linear moieties.
 A quick note to mention is the work corresponding to the hysteresis in the force profile (Figure 2) does not correlate to the bond energy. The work done in retracting the tip is approximated due to the linear behavior of deformation with being the force and being the displacement immediately before release. Using the results of Frisbie et al., normalized to the estimated 50 functional groups in contact, the work values are estimated as 39 eV, 0.25 eV, and 4.3 eV for , , and interactions, respectively. Roughly, intermolecular bond energies can be calculated by: being the boiling point. According to this, for
A quick note to mention is the work corresponding to the hysteresis in the force profile (Figure 2) does not correlate to the bond energy. The work done in retracting the tip is approximated due to the linear behavior of deformation with being the force and being the displacement immediately before release. Using the results of Frisbie et al., normalized to the estimated 50 functional groups in contact, the work values are estimated as 39 eV, 0.25 eV, and 4.3 eV for , , and interactions, respectively. Roughly, intermolecular bond energies can be calculated by: being the boiling point. According to this, for formic acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid. It has the chemical formula HCOOH and structure . This acid is an important intermediate in chemical synthesis and occurs naturally, most notably in some an ...
, , and 9.73 meV for methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
, , each value being about 3 orders of magnitude smaller than the experiment might suggest. Even if surface passivation with were considered (discussed below), the large error seems irrecoverable. The strongest hydrogen bonds are at most ~1 eV in energy. This strongly implies that the cantilever has a force constant smaller than or on the order of that for bond interactions and, therefore, it cannot be treated as perfectly rigid. This does open an avenue for increasing the usefulness of CFM if stiffer cantilevers can be used while still maintaining force resolution.
Frictional force mapping
Chemical interactions can also be used to map prepatterned substrates with varying functionalities (see Figure 3). Scanning of a surface having varying hydrophobicity with a tip having no functional groups attached would produce an image with no contrast because the surface is morphologically featureless (simple AFM operation). Functionalizing a tip to be hydrophilic would cause the cantilever to bend when the tip scans across hydrophilic portions of the substrate due to strong tip-substrate interactions. This is detected by laser deflection in aposition sensitive detector
Position often refers to:
* Position (geometry), the spatial location (rather than orientation) of an entity
* Position, a job or occupation
Position may also refer to:
Games and recreation
* Position (poker), location relative to the dealer
* ...
, thereby producing a chemical profile image of the surface. Generally, a brighter area would correspond to a greater amplitude of deflection, so stronger bonding corresponds to lighter areas of a CFM image map. When the cantilever functionalization is switched such that the tip is bent when encountering hydrophobic areas of the substrate instead, the complementary image is observed.
 Frictional force response to the amount of perpendicular load applied by the tip on to the substrate is shown in Figure 4. Increasing tip-substrate interactions produce a steeper slope, as one would expect. Of experimental importance is the fact that contrast between different functionalities on the surface may be enhanced with an application of greater perpendicular force. Of course, this comes at the cost of potential damage to the substrate.
Frictional force response to the amount of perpendicular load applied by the tip on to the substrate is shown in Figure 4. Increasing tip-substrate interactions produce a steeper slope, as one would expect. Of experimental importance is the fact that contrast between different functionalities on the surface may be enhanced with an application of greater perpendicular force. Of course, this comes at the cost of potential damage to the substrate.
Ambient: measurements in liquids
Capillary force is a major problem in tensile force measurements since it effectively strengthens the tip-surface interaction. It is usually caused by adsorbed moisture on substrates from ambient environment. To eliminate this additional force, measurements in liquids can be conducted. With X-terminated tip and substrate in liquid L, the addition to Fad is calculated using Eq 1 with WXLX = 2γLL; that is, the extra force comes from the attraction of liquid molecules to each other. This is ~10 pN for EtOH, which still allows for the observation of even the weakest polar/nonpolar interactions (~20 pN). The choice of liquid is dependent on which interactions are of interest. When the solvent is immiscible with functional groups, larger than usual tip-surface bonding exists. Therefore, organic solvents are appropriate for studying van der Waals and hydrogen bonding, while electrolytes are best for probing hydrophobic and electrostatic forces.Applications in nanoscience
 A biological implementation of CFM at the nanoscale level is the unfolding of proteins with functionalized tip and surface (see Figure 5). Due to the increased contact area, the tip and the surface act as anchors holding protein bundles while they separate. As uncoiling ensues, the force required jumps, indicating various stages of uncoiling: (1) separation into bundles, (2) bundle separation into domains of crystalline protein held together by van der Waals forces, and (3) linearization of the protein upon overcoming the secondary bonding. Information on the internal structure of these complex proteins, as well as a better understanding of constituent interactions are provided with this method.
A second consideration is one that takes advantage of unique nanoscale materials properties. The high
A biological implementation of CFM at the nanoscale level is the unfolding of proteins with functionalized tip and surface (see Figure 5). Due to the increased contact area, the tip and the surface act as anchors holding protein bundles while they separate. As uncoiling ensues, the force required jumps, indicating various stages of uncoiling: (1) separation into bundles, (2) bundle separation into domains of crystalline protein held together by van der Waals forces, and (3) linearization of the protein upon overcoming the secondary bonding. Information on the internal structure of these complex proteins, as well as a better understanding of constituent interactions are provided with this method.
A second consideration is one that takes advantage of unique nanoscale materials properties. The high aspect ratio
The aspect ratio of a geometry, geometric shape is the ratio of its sizes in different dimensions. For example, the aspect ratio of a rectangle is the ratio of its longer side to its shorter side—the ratio of width to height, when the rectangl ...
of carbon nanotubes
A carbon nanotube (CNT) is a tube made of carbon with a diameter in the nanometre range (nanoscale). They are one of the allotropes of carbon. Two broad classes of carbon nanotubes are recognized:
* ''Single-walled carbon nanotubes'' (''SWC ...
(easily >1000) is exploited to image surfaces with deep features. The use of the carbon material broadens the functionalization chemistry since there are countless routes to chemical modification of nanotube sidewalls (e.g. with diazonium, simple alkyls, hydrogen, ozone/oxygen, and amines). Multiwall nanotubes are typically used for their rigidity. Because of their approximately planar ends, one can estimate the number of functional groups that are in contact with the substrate knowing tube diameter and number of walls, which helps in determining single moiety tensile properties. Certainly, this method has obvious implications in tribology
Tribology is the science and engineering of understanding friction, lubrication and wear phenomena for interacting surfaces in relative Motion (physics), motion. It is highly interdisciplinary, drawing on many academic fields, including physics, c ...
as well.
References
{{SPM2 Microscopy Scanning probe microscopy