Cesium Fluoride on:
[Wikipedia]
[Google]
[Amazon]
Caesium fluoride or cesium fluoride is an
 Caesium fluoride can be prepared by the reaction of
Caesium fluoride can be prepared by the reaction of
.
www.hazard.com
.'' MSDS Date: April 27, 1993. Retrieved on September 7, 2007. Contact with acid should be avoided, as this forms highly toxic/corrosive
."
www.jtbaker.com
'' MSDS Date: January 16, 2006. Retrieved on September 7, 2007.
inorganic compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemi ...
with the formula CsF and it is a hygroscopic
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance ...
white salt. Caesium fluoride can be used in organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
as a source of the fluoride anion. Caesium
Caesium ( IUPAC spelling) (or cesium in American English) is a chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only five elemental metals that ...
also has the highest electropositivity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
of all non-radioactive elements and fluorine has the highest electronegativity of all known elements.
Synthesis and properties
 Caesium fluoride can be prepared by the reaction of
Caesium fluoride can be prepared by the reaction of caesium hydroxide
Caesium hydroxide is a strong base (pKa= 15.76) containing the highly reactive alkali metal caesium, much like the other alkali metal hydroxides such as sodium hydroxide and potassium hydroxide. Caesium hydroxide is corrosive enough to quickly ...
(CsOH) with hydrofluoric acid
Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colourless, acidic and highly corrosive. It is used to make most fluorine-containing compounds; examples include the commonly used pharmaceutical antidepr ...
(HF) and the resulting salt can then be purified by recrystallization. The reaction is shown below:
:CsOH + HF → CsF + H2O
Using the same reaction, another way to create caesium fluoride is to treat caesium carbonate
Caesium carbonate or cesium carbonate is a white crystalline solid compound. Caesium carbonate has a high solubility in polar solvents such as water, alcohol and DMF. Its solubility is higher in organic solvents compared to other carbonates like ...
(Cs2CO3) with hydrofluoric acid and again, the resulting salt can then be purified by recrystallization. The reaction is shown below:
:Cs2CO3 + 2 HF → 2 CsF + H2O + CO2
CsF is more soluble than sodium fluoride
Sodium fluoride (NaF) is an inorganic compound with the formula . It is used in trace amounts in the fluoridation of drinking water, in toothpaste, in metallurgy, and as a flux. It is a colorless or white solid that is readily soluble in water. ...
or potassium fluoride
Potassium fluoride is the chemical compound with the formula KF. After hydrogen fluoride, KF is the primary source of the fluoride ion for applications in manufacturing and in chemistry. It is an alkali halide and occurs naturally as the rare ...
in organic solvents. It is available in its anhydrous form, and if water has been absorbed, it is easy to dry by heating at 100 °C for two hours ''in vacuo
A vacuum is a space devoid of matter. The word is derived from the Latin adjective ''vacuus'' for "vacant" or " void". An approximation to such vacuum is a region with a gaseous pressure much less than atmospheric pressure. Physicists often di ...
''. CsF reaches a vapor pressure
Vapor pressure (or vapour pressure in English-speaking countries other than the US; see spelling differences) or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phase ...
of 1 kilopascal
The pascal (symbol: Pa) is the unit of pressure in the International System of Units (SI), and is also used to quantify internal pressure, stress, Young's modulus, and ultimate tensile strength. The unit, named after Blaise Pascal, is defined ...
at 825 °C, 10 kPa at 999 °C, and 100 kPa at 1249 °C.
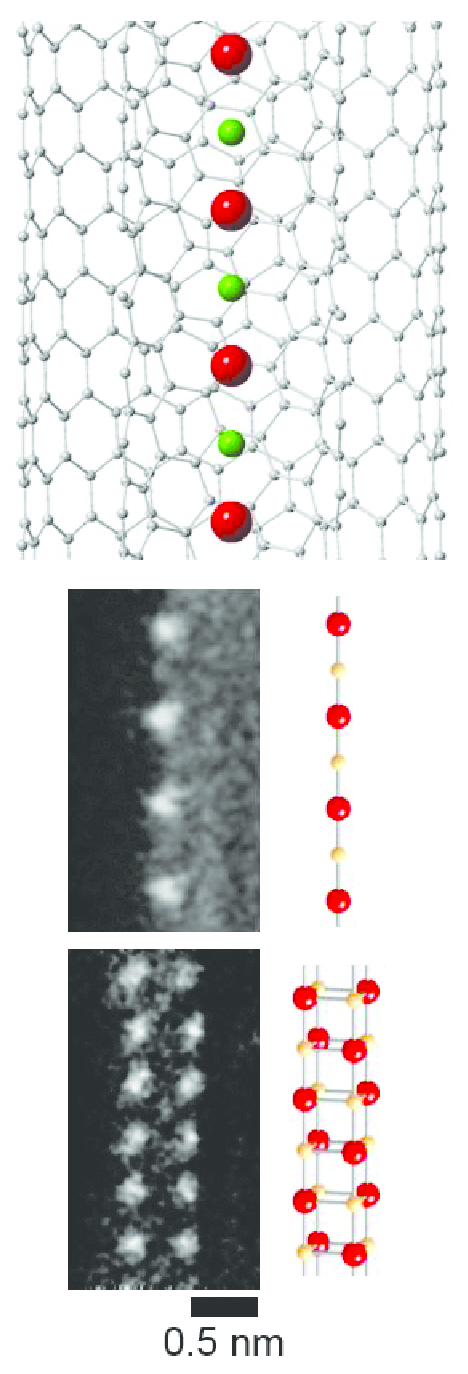
CsF chains with a thickness as small as one or two atoms can be grown inside carbon nanotube
A scanning tunneling microscopy image of a single-walled carbon nanotube
Rotating single-walled zigzag carbon nanotube
A carbon nanotube (CNT) is a tube made of carbon with diameters typically measured in nanometers.
''Single-wall carbon nan ...
s.
Structure
Caesium fluoride has the halite structure, which means that the Cs+ and F− pack in acubic closest packed
In geometry, close-packing of equal spheres is a dense arrangement of congruent spheres in an infinite, regular arrangement (or lattice). Carl Friedrich Gauss proved that the highest average density – that is, the greatest fraction of space occu ...
array as do Na+ and Cl− in sodium chloride.
Applications in organic synthesis
Being highly dissociated, CsF is a more reactive source of fluoride than related salts. CsF is an alternative totetra-n-butylammonium fluoride
Tetra-''n''-butylammonium fluoride, commonly abbreviated to TBAF and ''n''-Bu4NF, is a quaternary ammonium salt with the chemical formula (CH3CH2CH2CH2)4N+F−. It is commercially available as the white solid trihydrate and as a solution in tetra ...
(TBAF) and TAS-fluoride (TASF).
As a base
As with other soluble fluorides, CsF is moderately basic, because HF is aweak acid
Acid strength is the tendency of an acid, symbolised by the chemical formula HA, to dissociate into a hydron (chemistry), proton, H+, and an anion, A-. The Dissociation (chemistry), dissociation of a strong acid in solution is effectively comple ...
. The low nucleophilicity
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
of fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an inorganic, monatomic anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts ...
means it can be a useful base in organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clay ...
. CsF gives higher yields in Knoevenagel condensation
In organic chemistry, the Knoevenagel condensation () reaction is a type of chemical reaction named after German chemist Emil Knoevenagel. It is a modification of the aldol condensation.
A Knoevenagel condensation is a nucleophilic addition of ...
reactions than KF or NaF.
Formation of Cs-F bonds
Caesium fluoride serves as a source of fluoride inorganofluorine chemistry
Organofluorine chemistry describes the chemistry of the organofluorines, organic compounds that contain the carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, refri ...
. Similarly to potassium
Potassium is the chemical element with the symbol K (from Neo-Latin '' kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmosp ...
fluoride, CsF reacts with hexafluoroacetone
Hexafluoroacetone (HFA) is a chemical compound with the formula (CF3)2CO. It is structurally similar to acetone; however, its reactivity is markedly different. It a colourless, hygroscopic, nonflammable, highly reactive gas characterized by a mus ...
to form a stable perfluoroalkoxide salt. It will convert electron-deficient Electron deficiency (and electron-deficient) is jargon that is used in two contexts: species that violate the octet rule because they have too few valence electrons and species that happen to follow the octet rule but have electron-acceptor properti ...
aryl chloride In organic chemistry, an aryl halide (also known as haloarene) is an aromatic compound in which one or more hydrogen atoms, directly bonded to an aromatic ring are replaced by a halide. The haloarene are different from haloalkanes because they exhi ...
s to aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as ...
fluorides (Halex process
In chemistry, the Halex process is used to convert aromatic chlorides to the corresponding aromatic fluorides. The process entails ''Hal''ide ''ex''change, hence the name. The reaction conditions call for hot (150-250 °C) solution of the aryl ch ...
), although potassium fluoride is more commonly used.
Deprotection agent
Due to the strength of the Si– F bond, fluoride is useful fordesilylation
Silylation is the introduction of one or more (usually) substituted silyl groups (R3Si) to a molecule. The process is the basis of organosilicon chemistry.
Of organic compounds
Alcohols, carboxylic acids, amines, thiols, and phosphates can be sil ...
reactions, i.e. cleavage of Si-O bonds in organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
. CsF is commonly used for such reactions. Solutions of caesium fluoride in THF or DMF attack a wide variety of organosilicon
Organosilicon compounds are organometallic compounds containing carbon–silicon bonds. Organosilicon chemistry is the corresponding science of their preparation and properties. Most organosilicon compounds are similar to the ordinary organic c ...
compounds to produce an organosilicon fluoride and a carbanion
In organic chemistry, a carbanion is an anion in which carbon is trivalent (forms three bonds) and bears a formal negative charge (in at least one significant resonance form).
Formally, a carbanion is the conjugate base of a carbon acid:
:R3CH ...
, which can then react with electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that ca ...
s, for example:
:
Precautions
Like other soluble fluorides, CsF is moderately toxic.MSDS Listing for cesium fluoride.
www.hazard.com
.'' MSDS Date: April 27, 1993. Retrieved on September 7, 2007. Contact with acid should be avoided, as this forms highly toxic/corrosive
hydrofluoric acid
Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colourless, acidic and highly corrosive. It is used to make most fluorine-containing compounds; examples include the commonly used pharmaceutical antidepr ...
. The caesium ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
(Cs+) and caesium chloride
Caesium chloride or cesium chloride is the inorganic compound with the formula Cs Cl. This colorless salt is an important source of caesium ions in a variety of niche applications. Its crystal structure forms a major structural type where each c ...
are generally not considered toxic.MSDS Listing for cesium chloride."
www.jtbaker.com
'' MSDS Date: January 16, 2006. Retrieved on September 7, 2007.
References
{{Fluorides Fluorides Caesium compounds Metal halides Alkali metal fluorides Rock salt crystal structure