Cerocene on:
[Wikipedia]
[Google]
[Amazon]
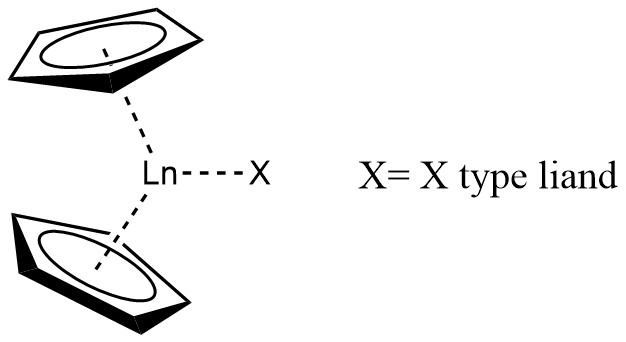 A lanthanocene is a type of
A lanthanocene is a type of
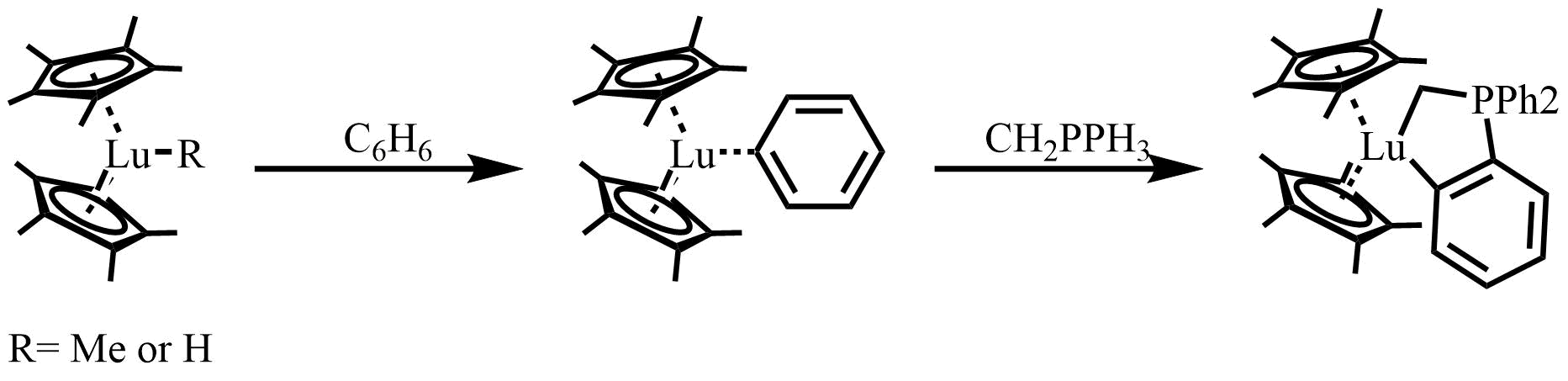 In 1983, Watson reported one of the first lanthanocene catalyzed C-H bond activation reactions. The active catalysts are lutetium-methyl or lutetium-hydride complexes, which react at room temperature in hydrocarbon solvents with benzene, pyridine, and the
In 1983, Watson reported one of the first lanthanocene catalyzed C-H bond activation reactions. The active catalysts are lutetium-methyl or lutetium-hydride complexes, which react at room temperature in hydrocarbon solvents with benzene, pyridine, and the  Mechanism for hydrogenation is shown below, where the active catalyst is generated by
Mechanism for hydrogenation is shown below, where the active catalyst is generated by 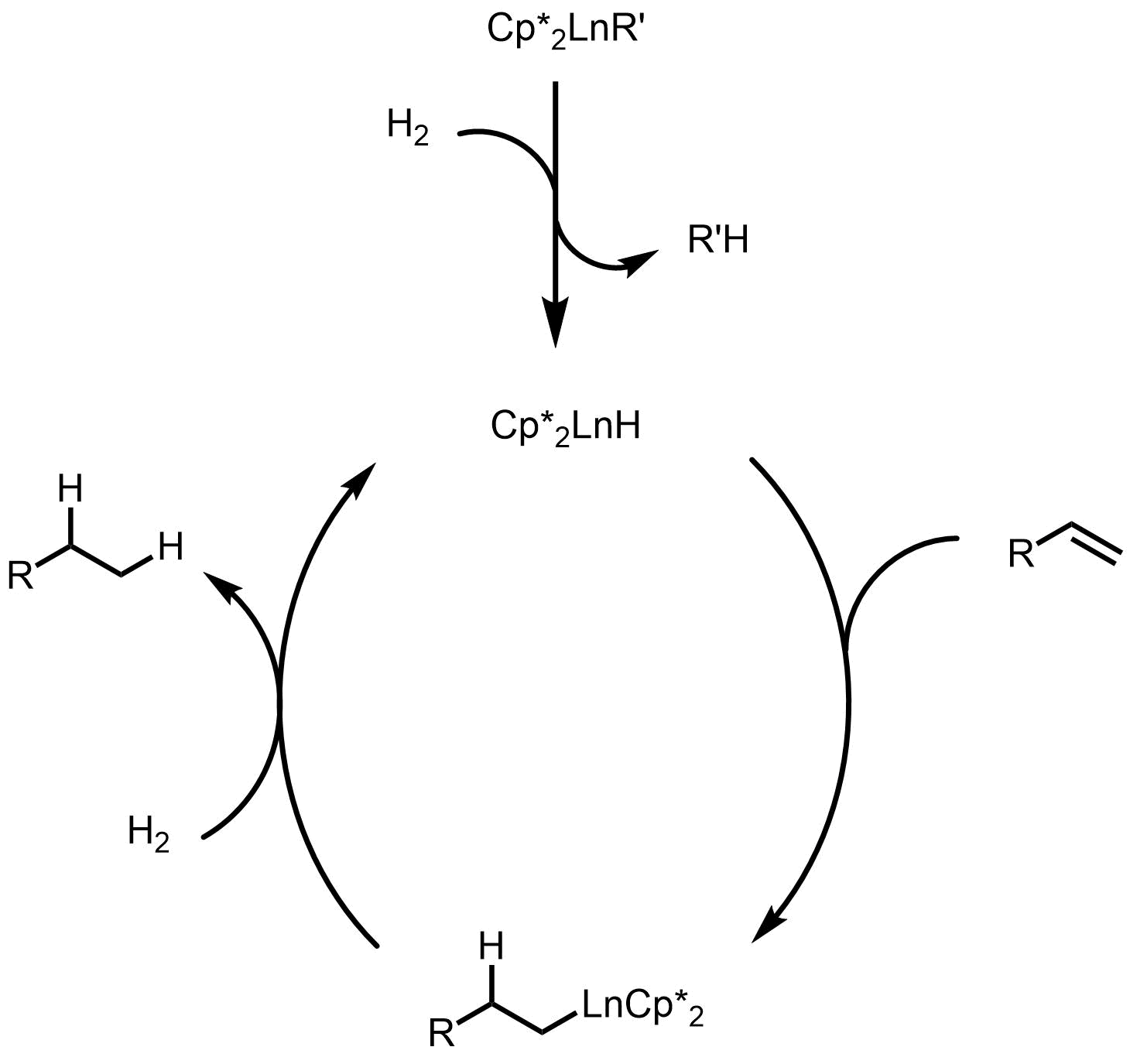
 Yet, as the large cyclopentadienyl ligand hinders the metal center, reactions with substituted alkenes are inhibited. Two general methods are used to overcome this difficulty. One is to increase the size of metal by incorporating lanthanides with larger ionic radii. Due to the
Yet, as the large cyclopentadienyl ligand hinders the metal center, reactions with substituted alkenes are inhibited. Two general methods are used to overcome this difficulty. One is to increase the size of metal by incorporating lanthanides with larger ionic radii. Due to the
 A lanthanocene is a type of
A lanthanocene is a type of metallocene
A metallocene is a compound typically consisting of two cyclopentadienyl anions (, abbreviated Cp) bound to a metal center (M) in the oxidation state II, with the resulting general formula Closely related to the metallocenes are the metallocene d ...
compound that contains an element from the lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and y ...
series. The most common lanthanocene complexes contain two cyclopentadienyl anions and an X type ligand, usually hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of ...
or alkyl ligand.
History
In 1954, Wilkinson and Birmingham described the tris(cyclopentadienyl)lanthanide complex, Ln(C5H5)3 (Ln = La, Ce, Pr, Nd, Sm). However, due to the highly moisture and oxygen sensitive character of organolanthanide compounds, as well as the incapability of the separation of simple alkyl and aryl derivatives of the type LnR3, this area of organomemtallic chemistry experienced a period of relative stagnation for two decades before lanthanocene complexes were prepared for some of the later lanthanides (Ln = Gd, Er, Yb, Lu).Synthesis
The synthesis part will focus on lanthanide(III) metallocene complexes that contain Ln-C bonds, which are widely prepared from the corresponding Ln-Cl precursors as shown. : + LiR → + LiCl ::Ln = Y, Nd, Sm, Dy, Er, Tm, Yb, Lu ::R = Me, Et, iPr, nBu, tBu, CH2tBu, , , Ph, The synthetic route leading to lanthanocene chlorides are summarized: : + 2 → + 2 MCl ::M = Na, Ti : + → + + : + HCl → +Reactions
With the large 4f orbitals, lanthanide elements display properties significantly different from the common d-block transition metals. The large ionic radii limits the extent to which 4f orbitals can overlap with ligands, but at the same time allows the organolanthanide complexes to attain higher coordination numbers. As such,cyclopentadienyl anion
In chemistry, the cyclopentadienyl anion or cyclopentadienide is an aromatic species with a formula of and abbreviated as Cp−. It is formed from the deprotonation of the molecule cyclopentadiene.
Properties
The cyclopentadienyl anion ...
s (abbreviated Cp) are typically used to occupy the unsaturated site as well as stabilize the metal complex. Some of the alkyl and hydride lanthanocene complexes exhibit unique activities towards C-H bond activation, alkene functionalization, and carbonyl activation.
 In 1983, Watson reported one of the first lanthanocene catalyzed C-H bond activation reactions. The active catalysts are lutetium-methyl or lutetium-hydride complexes, which react at room temperature in hydrocarbon solvents with benzene, pyridine, and the
In 1983, Watson reported one of the first lanthanocene catalyzed C-H bond activation reactions. The active catalysts are lutetium-methyl or lutetium-hydride complexes, which react at room temperature in hydrocarbon solvents with benzene, pyridine, and the ylide An ylide or ylid () is a neutral dipolar molecule containing a formally negatively charged atom (usually a carbanion) directly attached to a heteroatom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both ato ...
CH2PPh3 to give stable, isolatable products.
Studies have shown that organolanthanides are extraordinary catalysts for hydrofunctionalization reactions including hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate org ...
, hydrosilylation Hydrosilylation, also called catalytic hydrosilation, describes the addition of Si-H bonds across unsaturated bonds."Hydrosilylation A Comprehensive Review on Recent Advances" B. Marciniec (ed.), Advances in Silicon Science, Springer Science, 2009 ...
, hydroboration
In organic chemistry, hydroboration refers to the addition of a hydrogen- boron bond to certain double and triple bonds involving carbon (, , , and ). This chemical reaction is useful in the organic synthesis of organic compounds.
Hydroboration ...
, hydroamination
In organic chemistry, hydroamination is the addition of an bond of an amine across a carbon-carbon multiple bond of an alkene, alkyne, diene, or allene. In the ideal case, hydroamination is atom economical and green. Amines are common in fine-c ...
, etc. Examples for each type have shown below.
 Mechanism for hydrogenation is shown below, where the active catalyst is generated by
Mechanism for hydrogenation is shown below, where the active catalyst is generated by sigma-bond metathesis In organometallic chemistry, sigma-bond metathesis is a chemical reaction wherein a metal-ligand sigma bond undergoes metathesis (exchange of parts) with the sigma bond in some reagent. The reaction is illustrated by the exchange of lutetium(III) ...
, followed by olefin insertion and another sigma-bond metathesis to regenerate the catalyst. This is also the mechanism for other hydrofunctionalization.

 Yet, as the large cyclopentadienyl ligand hinders the metal center, reactions with substituted alkenes are inhibited. Two general methods are used to overcome this difficulty. One is to increase the size of metal by incorporating lanthanides with larger ionic radii. Due to the
Yet, as the large cyclopentadienyl ligand hinders the metal center, reactions with substituted alkenes are inhibited. Two general methods are used to overcome this difficulty. One is to increase the size of metal by incorporating lanthanides with larger ionic radii. Due to the lanthanide contraction
The lanthanide contraction is the greater-than-expected decrease in atomic radii/ ionic radii of the elements in the lanthanide series from atomic number 57, lanthanum, to 71, lutetium, which results in smaller than otherwise expected atomic radi ...
, this means replacing the late lanthanides with the early lanthanides. Another method is to decrease the size of the ligand by manipulating the geometry of ligands and substitutions on ligands. For example, a hinged or ansa-bridged cyclopentadienyl ligand could be used to pull ligands closer to each other, and hence creating more open access to the metal center as shown on the right.
See also
*Actinocene
Actinocenes are a family of organoactinide compounds consisting of metallocenes containing elements from the actinide series. They typically have a sandwich structure with two dianionic cyclooctatetraenyl ligands (COT2-, which is ) bound to an ...
References
{{Reflist Metallocenes Lanthanide compounds