Carboxylate Salt on:
[Wikipedia]
[Google]
[Amazon]

 In
In
RCOOH + NaOH -> RCOONa + H2O
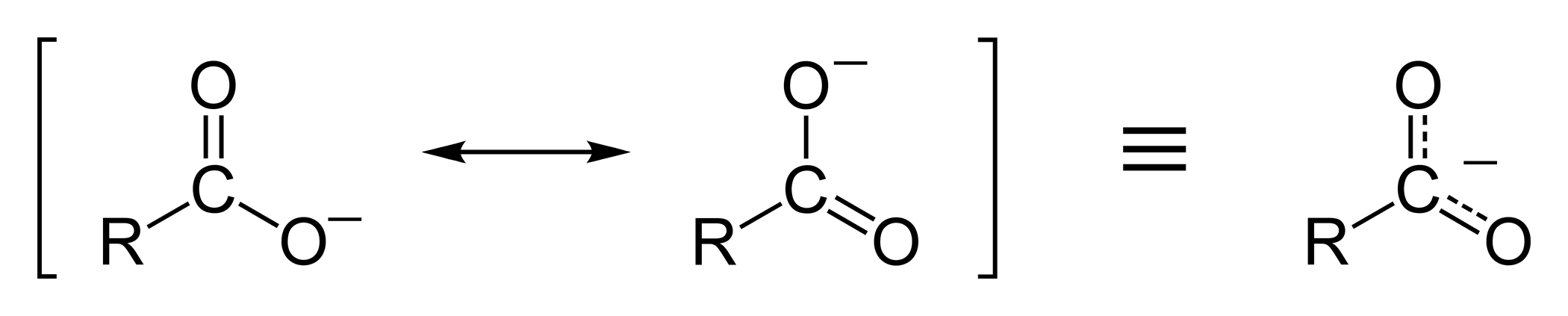 This delocalization of the electron cloud means that both of the oxygen atoms are less strongly negatively charged; the positive proton is therefore less strongly attracted back to the carboxylate group once it has left; hence, the carboxylate ion is more stable and less basic as a result of
This delocalization of the electron cloud means that both of the oxygen atoms are less strongly negatively charged; the positive proton is therefore less strongly attracted back to the carboxylate group once it has left; hence, the carboxylate ion is more stable and less basic as a result of
 The nucleophilicity of carboxylate ions are much weaker than that of
The nucleophilicity of carboxylate ions are much weaker than that of



 In
In organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clay ...
, a carboxylate is the conjugate base
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid donates a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as in the reverse reaction it loses a ...
of a carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxyl ...
, (or ). It is an ion with negative charge.
Carboxylate salts are salts
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively ...
that have the general formula , where M is a metal and ''n'' is 1, 2,...; ''carboxylate esters'' have the general formula (or ). R and R′ are organic groups; R′ ≠ H.
Synthesis
Carboxylate ions can be formed by deprotonation of carboxylic acids. Such acids typically have p''K''a of less than 5, meaning that they can be deprotonated by many bases, such assodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alkal ...
or sodium bicarbonate.
:Resonance stabilization of the carboxylate ion
Carboxylic acids easily dissociate into a carboxylate anion and a positively charged hydrogen ion (proton), much more readily than alcohols do (into an alkoxide ion and a proton), because the carboxylate ion is stabilized byresonance
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an oscillat ...
. The negative charge that is left after deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju. ...
of the carboxyl group is delocalized between the two electronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
oxygen atoms in a resonance structure. If the R group is an electron-withdrawing group (such as –CF3), the basicity of the carboxylate will be further weakened.
: This delocalization of the electron cloud means that both of the oxygen atoms are less strongly negatively charged; the positive proton is therefore less strongly attracted back to the carboxylate group once it has left; hence, the carboxylate ion is more stable and less basic as a result of
This delocalization of the electron cloud means that both of the oxygen atoms are less strongly negatively charged; the positive proton is therefore less strongly attracted back to the carboxylate group once it has left; hence, the carboxylate ion is more stable and less basic as a result of resonance stabilization
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or ...
of the negative charge. In contrast, an alkoxide ion, once formed, would have a strong negative charge localized on its lone oxygen atom, which would strongly attract any nearby protons (indeed, alkoxides are very strong bases). Because of resonance stabilization, carboxylic acids have much lower p''K''a values (and are therefore stronger acids) than alcohols
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which i ...
. For example, the p''K''a value of acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main componen ...
is 4.8, while ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a h ...
has a p''K''a of 16. Hence acetic acid is a much stronger acid than ethanol. This in turn means that for equimolar solutions of a carboxylic acid and an alcohol, the carboxylic acid would have a much lower pH.
Reactions
Nucleophilic substitution
Carboxylate ions are good nucleophiles. They react with alkyl halides to formester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ...
. The following reaction shows the reaction mechanism.
 The nucleophilicity of carboxylate ions are much weaker than that of
The nucleophilicity of carboxylate ions are much weaker than that of hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water ...
and alkoxide ions, but stronger than halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a f ...
anions (in a polar aprotic solvent, though there are other effects such as solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solub ...
of the ion).
Reduction
Unlike the reduction of ester, the reduction of carboxylate is different, due to the lack of theleaving group In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited ...
and the relatively electron-rich carbon atom (due to the negative charge on the oxygen atoms). With a small amount of acid, the reaction occurs with lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula Li Al H4. It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic ...
by changing the LAH into the Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
AlH3 in the process, converting the oxyanion to 4 Al–O bonds.
Examples
This list is for cases where there is a separate article for the anion or its derivatives. All other organic acids should be found at their parent carboxylic acid. *Formate
Formate ( IUPAC name: methanoate) is the conjugate base of formic acid. Formate is an anion () or its derivatives such as ester of formic acid. The salts and esters are generally colorless.Werner Reutemann and Heinz Kieczka "Formic Acid" in ...
ion, HCOO−
* Acetate
An acetate is a salt formed by the combination of acetic acid with a base (e.g. alkaline, earthy, metallic, nonmetallic or radical base). "Acetate" also describes the conjugate base or ion (specifically, the negatively charged ion called ...
ion, CH3COO−
* Methanetetracarboxylate ion, C(COO−)4
* Oxalate
Oxalate (IUPAC: ethanedioate) is an anion with the formula C2O42−. This dianion is colorless. It occurs naturally, including in some foods. It forms a variety of salts, for example sodium oxalate (Na2C2O4), and several esters such as dimethyl ...
ion,
See also
*Carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxyl ...
References
{{Reflist Anions...........