Carbonate Alkalinity on:
[Wikipedia]
[Google]
[Amazon]
A carbonate is a
 This structure is incompatible with the observed symmetry of the ion, which implies that the three bonds are the same length and that the three oxygen atoms are equivalent. As in the case of the
This structure is incompatible with the observed symmetry of the ion, which implies that the three bonds are the same length and that the three oxygen atoms are equivalent. As in the case of the  This resonance can be summarized by a model with fractional bonds and
This resonance can be summarized by a model with fractional bonds and 
Carbonate/bicarbonate/carbonic acid equilibrium in water: pH of solutions, buffer capacity, titration and species distribution vs. pH computed with a free spreadsheet
* {{Authority control Carbon oxyanions Oxocarbon anions
salt
In common usage, salt is a mineral composed primarily of sodium chloride (NaCl). When used in food, especially in granulated form, it is more formally called table salt. In the form of a natural crystalline mineral, salt is also known as r ...
of carbonic acid
Carbonic acid is a chemical compound with the chemical formula . The molecule rapidly converts to water and carbon dioxide in the presence of water. However, in the absence of water, it is quite stable at room temperature. The interconversion ...
, (), characterized by the presence of the carbonate ion, a polyatomic ion
A polyatomic ion (also known as a molecular ion) is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that usually has a net charge that is not zero, or in special c ...
with the formula . The word "carbonate" may also refer to a carbonate ester
In organic chemistry, a carbonate ester (organic carbonate or organocarbonate) is an ester of carbonic acid. This functional group consists of a carbonyl group flanked by two alkoxy groups. The general structure of these carbonates is and they a ...
, an organic compound containing the carbonate group .
The term is also used as a verb, to describe carbonation
Carbonation is the chemical reaction of carbon dioxide to give carbonates, bicarbonates, and carbonic acid. In chemistry, the term is sometimes used in place of carboxylation, which refers to the formation of carboxylic acids.
In inorganic che ...
: the process of raising the concentrations of carbonate and bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial bioche ...
ions in water to produce carbonated water
Carbonated water is water containing dissolved carbon dioxide gas, either artificially injected under pressure, or occurring due to natural geological processes. Carbonation causes small bubbles to form, giving the water an effervescent quali ...
and other carbonated beverageseither by the addition of carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
gas under pressure or by dissolving carbonate or bicarbonate salts into the water.
In geology
Geology (). is a branch of natural science concerned with the Earth and other astronomical objects, the rocks of which they are composed, and the processes by which they change over time. Modern geology significantly overlaps all other Earth ...
and mineralogy
Mineralogy is a subject of geology specializing in the scientific study of the chemistry, crystal structure, and physical (including optical mineralogy, optical) properties of minerals and mineralized artifact (archaeology), artifacts. Specific s ...
, the term "carbonate" can refer both to carbonate minerals
Carbonate minerals are those minerals containing the carbonate ion, .
Carbonate divisions Anhydrous carbonates
*Calcite group: trigonal
**Calcite CaCO3
**Gaspéite (Ni,Mg,Fe2+)CO3
**Magnesite MgCO3
**Otavite CdCO3
**Rhodochrosite MnCO3
**Sider ...
and carbonate rock
Carbonate rocks are a class of sedimentary rocks composed primarily of carbonate minerals. The two major types are limestone, which is composed of calcite or aragonite (different crystal forms of CaCO3), and Dolomite (rock), dolomite rock (also kn ...
(which is made of chiefly carbonate minerals), and both are dominated by the carbonate ion, . Carbonate minerals are extremely varied and ubiquitous in chemically precipitated sedimentary rock
Sedimentary rocks are types of rock (geology), rock formed by the cementation (geology), cementation of sediments—i.e. particles made of minerals (geological detritus) or organic matter (biological detritus)—that have been accumulated or de ...
. The most common are calcite
Calcite is a Carbonate minerals, carbonate mineral and the most stable Polymorphism (materials science), polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on ...
or calcium carbonate
Calcium carbonate is a chemical compound with the chemical formula . It is a common substance found in Rock (geology), rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skel ...
, , the chief constituent of limestone
Limestone is a type of carbonate rock, carbonate sedimentary rock which is the main source of the material Lime (material), lime. It is composed mostly of the minerals calcite and aragonite, which are different Polymorphism (materials science) ...
(as well as the main component of mollusc
Mollusca is a phylum of protostome, protostomic invertebrate animals, whose members are known as molluscs or mollusks (). Around 76,000 extant taxon, extant species of molluscs are recognized, making it the second-largest animal phylum ...
shells and coral
Corals are colonial marine invertebrates within the subphylum Anthozoa of the phylum Cnidaria. They typically form compact Colony (biology), colonies of many identical individual polyp (zoology), polyps. Coral species include the important Coral ...
skeletons); dolomite, a calcium-magnesium carbonate ; and siderite
Siderite is a mineral composed of iron(II) carbonate (FeCO3). Its name comes from the Ancient Greek word (), meaning "iron". A valuable iron ore, it consists of 48% iron and lacks sulfur and phosphorus. Zinc, magnesium, and manganese commonly ...
, or iron(II) carbonate
Iron(II) carbonate, or ferrous carbonate, is a chemical compound with formula , that occurs naturally as the mineral siderite. At ordinary ambient temperatures, it is a green-brown ionic solid consisting of iron(II) cations and carbonate anion ...
, , an important iron ore
Iron ores are rocks and minerals from which metallic iron can be economically extracted. The ores are usually rich in iron oxides and vary in color from dark grey, bright yellow, or deep purple to rusty red. The iron is usually found in the f ...
. Sodium carbonate
Sodium carbonate (also known as washing soda, soda ash, sal soda, and soda crystals) is the inorganic compound with the formula and its various hydrates. All forms are white, odourless, water-soluble salts that yield alkaline solutions in water ...
("soda" or "natron"), , and potassium carbonate
Potassium carbonate is the inorganic compound with the formula . It is a white salt, which is soluble in water and forms a strongly alkaline solution. It is deliquescent, often appearing as a damp or wet solid. Potassium carbonate is mainly used ...
("potash"), , have been used since antiquity for cleaning and preservation, as well as for the manufacture of glass
Glass is an amorphous (non-crystalline solid, non-crystalline) solid. Because it is often transparency and translucency, transparent and chemically inert, glass has found widespread practical, technological, and decorative use in window pane ...
. Carbonates are widely used in industry, such as in iron smelting, as a raw material for Portland cement
Portland cement is the most common type of cement in general use around the world as a basic ingredient of concrete, mortar (masonry), mortar, stucco, and non-specialty grout. It was developed from other types of hydraulic lime in England in th ...
and lime manufacture, in the composition of ceramic glaze
Ceramic glaze, or simply glaze, is a glassy coating on ceramics. It is used for decoration, to ensure the item is impermeable to liquids and to minimize the adherence of pollutants.
Glazing renders earthenware impermeable to water, sealing th ...
s, and more. New applications of alkali metal carbonates include: thermal energy storage, catalysis and electrolyte both in fuel cell technology as well as in electrosynthesis of in aqueous media.
Structure and bonding
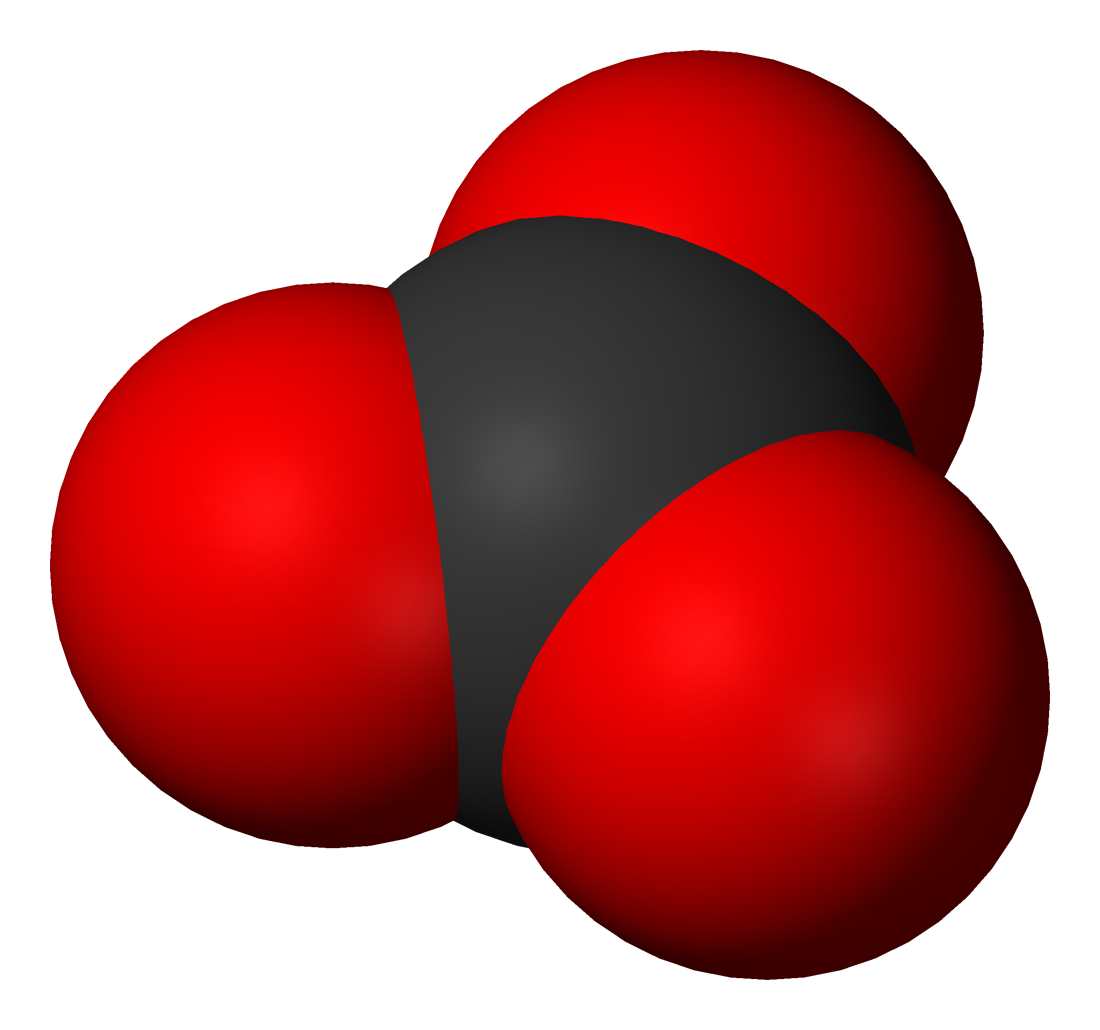
The carbonate ion is the simplest oxocarbon anion. It consists of onecarbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
atom surrounded by three oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
atoms, in a trigonal planar arrangement, with ''D''3h molecular symmetry
In chemistry, molecular symmetry describes the symmetry present in molecules and the classification of these molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can be used to predict or explai ...
. It has a molecular mass of 60.01 g/mol and carries a total formal charge
In chemistry, a formal charge (F.C. or ), in the covalent view of chemical bonding, is the hypothetical charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of rela ...
of −2. It is the conjugate base
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid gives a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as it loses a hydrogen ion in the reve ...
of the hydrogencarbonate (bicarbonate) ion, , which is the conjugate base of , carbonic acid
Carbonic acid is a chemical compound with the chemical formula . The molecule rapidly converts to water and carbon dioxide in the presence of water. However, in the absence of water, it is quite stable at room temperature. The interconversion ...
.
The Lewis structure
Lewis structuresalso called Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDs)are diagrams that show the chemical bond, bonding between atoms of a molecule, as well as the lone pairs of elec ...
of the carbonate ion has two (long) single bonds to negative oxygen atoms, and one short double bond to a neutral oxygen atom.
: This structure is incompatible with the observed symmetry of the ion, which implies that the three bonds are the same length and that the three oxygen atoms are equivalent. As in the case of the
This structure is incompatible with the observed symmetry of the ion, which implies that the three bonds are the same length and that the three oxygen atoms are equivalent. As in the case of the isoelectronic
Isoelectronicity is a phenomenon observed when two or more molecules have the same structure (positions and connectivities among atoms) and the same electronic configurations, but differ by what specific elements are at certain locations in th ...
nitrate
Nitrate is a polyatomic ion with the chemical formula . salt (chemistry), Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are solubility, soluble in wa ...
ion, the symmetry can be achieved by a resonance
Resonance is a phenomenon that occurs when an object or system is subjected to an external force or vibration whose frequency matches a resonant frequency (or resonance frequency) of the system, defined as a frequency that generates a maximu ...
among three structures:
: This resonance can be summarized by a model with fractional bonds and
This resonance can be summarized by a model with fractional bonds and delocalized
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref>
The term delocalization is general and can have slightly dif ...
charges:
:

Chemical properties
190px, Stalactites and stalagmites are carbonate minerals. Metal carbonates generally decompose on heating, liberating carbon dioxide leaving behind an oxide of the metal. This process is calledcalcination
Calcination is thermal treatment of a solid chemical compound (e.g. mixed carbonate ores) whereby the compound is raised to high temperature without melting under restricted supply of ambient oxygen (i.e. gaseous O2 fraction of air), generally f ...
, after ''calx'', the Latin name of quicklime or calcium oxide
Calcium oxide (formula: Ca O), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term '' lime'' connotes calcium-containing ...
, CaO, which is obtained by roasting limestone in a lime kiln
A lime kiln is a kiln used for the calcination of limestone (calcium carbonate) to produce the form of lime called ''quicklime'' (calcium oxide). The chemical equation for this reaction is: CaCO3 + heat → CaO + CO2
This reaction can tak ...
:
:
As illustrated by its affinity for , carbonate is a ligand for many metal cations. Transition metal carbonate and bicarbonate complexes feature metal ions covalently bonded to carbonate in a variety of bonding modes.
Lithium
Lithium (from , , ) is a chemical element; it has chemical symbol, symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard temperature and pressure, standard conditions, it is the least dense metal and the ...
, sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the peri ...
, potassium
Potassium is a chemical element; it has Symbol (chemistry), symbol K (from Neo-Latin ) and atomic number19. It is a silvery white metal that is soft enough to easily cut with a knife. Potassium metal reacts rapidly with atmospheric oxygen to ...
, rubidium
Rubidium is a chemical element; it has Symbol (chemistry), symbol Rb and atomic number 37. It is a very soft, whitish-grey solid in the alkali metal group, similar to potassium and caesium. Rubidium is the first alkali metal in the group to have ...
, caesium
Caesium (IUPAC spelling; also spelled cesium in American English) is a chemical element; it has Symbol (chemistry), symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only f ...
, and ammonium
Ammonium is a modified form of ammonia that has an extra hydrogen atom. It is a positively charged (cationic) polyatomic ion, molecular ion with the chemical formula or . It is formed by the protonation, addition of a proton (a hydrogen nucleu ...
carbonates are water-soluble salts, but carbonates of 2+ and 3+ ions are often poorly soluble in water. Of the insoluble metal carbonates, is important because, in the form of scale, it accumulates in and impedes flow through pipes. Hard water
Hard water is water that has a high mineral content (in contrast with "soft water"). Hard water is formed when water percolates through deposits of limestone, chalk or gypsum, which are largely made up of calcium and magnesium carbonates, bic ...
is rich in this material, giving rise to the need for infrastructural water softening
Water softening is the removal of calcium, magnesium, and certain other metal cations in hard water. The resulting soft water requires less soap for the same cleaning effort, as soap is not wasted bonding with calcium ions. Soft water also extend ...
.
Acidification of carbonates generally liberates carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
:
:
Thus, scale can be removed with acid.
In solution the equilibrium between carbonate, bicarbonate, carbon dioxide and carbonic acid is sensitive to pH, temperature, and pressure. Although di- and trivalent carbonates have low solubility, bicarbonate salts are far more soluble. This difference is related to the disparate lattice energies of solids composed of mono- vs dianions, as well as mono- vs dications.
In aqueous solution
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, also known as sodium chloride (NaCl), in water ...
, carbonate, bicarbonate, carbon dioxide, and carbonic acid participate in a dynamic equilibrium
In chemistry, a dynamic equilibrium exists once a reversible reaction occurs. Substances initially transition between the reactants and products at different rates until the forward and backward reaction rates eventually equalize, meaning the ...
. In strongly basic conditions, the carbonate ion predominates, while in weakly basic conditions, the bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial bioche ...
ion is prevalent. In more acid conditions, aqueous carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
, , is the main form, which, with water, , is in equilibrium with carbonic acidthe equilibrium lies strongly towards carbon dioxide. Thus sodium carbonate
Sodium carbonate (also known as washing soda, soda ash, sal soda, and soda crystals) is the inorganic compound with the formula and its various hydrates. All forms are white, odourless, water-soluble salts that yield alkaline solutions in water ...
is basic, sodium bicarbonate
Sodium bicarbonate ( IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda (or simply “bicarb” especially in the UK) is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cat ...
is weakly basic, while carbon dioxide itself is a weak acid.
Organic carbonates
Inorganic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
a carbonate can also refer to a functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
within a larger molecule that contains a carbon atom bound to three oxygen atoms, one of which is double bonded. These compounds are also known as organocarbonates or carbonate esters, and have the general formula , or . Important organocarbonates include dimethyl carbonate
Dimethyl carbonate (DMC) is an organic compound with the formula OC(OCH3)2. It is a colourless, flammable liquid. It is classified as a carbonate ester. This compound has found use as a methylating agent and as a co-solvent in lithium-ion ba ...
, the cyclic compounds ethylene carbonate
Ethylene carbonate (sometimes abbreviated EC) is the organic compound with the formula (CH2O)2CO. It is classified as the cyclic carbonate ester of ethylene glycol and carbonic acid. At room temperature (25 °C) ethylene carbonate is a tra ...
and propylene carbonate
Propylene carbonate (often abbreviated PC) is an organic compound with the formula C4H6O3. It is a cyclic carbonate ester derived from propylene glycol. This colorless and odorless liquid is useful as a polar, aprotic solvent. Propylene carbon ...
, and the phosgene replacement, triphosgene.
Buffer
Three reversible reactions control the pH balance of blood and act as abuffer
Buffer may refer to:
Science
* Buffer gas, an inert or nonflammable gas
* Buffer solution, a solution used to prevent changes in pH
* Lysis buffer, in cell biology
* Metal ion buffer
* Mineral redox buffer, in geology
Technology and engineeri ...
to stabilise it in the range 7.37–7.43:
#
#
#
Exhaled depletes , which in turn consumes , causing the equilibrium of the first reaction to try to restore the level of carbonic acid by reacting bicarbonate with a hydrogen ion, an example of Le Châtelier's principle. The result is to make the blood more alkaline (raise pH). By the same principle, when the pH is too high, the kidneys excrete bicarbonate () into urine as urea via the urea cycle
The urea cycle (also known as the ornithine cycle) is a cycle of biochemical reactions that produces urea (NH2)2CO from ammonia (NH3). Animals that use this cycle, mainly amphibians and mammals, are called ureotelic.
The urea cycle converts highl ...
(or Krebs–Henseleit ornithine cycle). By removing the bicarbonate, more is generated from carbonic acid (), which comes from produced by cellular respiration
Cellular respiration is the process of oxidizing biological fuels using an inorganic electron acceptor, such as oxygen, to drive production of adenosine triphosphate (ATP), which stores chemical energy in a biologically accessible form. Cell ...
.
Crucially, a similar buffer operates in the oceans. It is a major factor in climate change and the long-term carbon cycle, due to the large number of marine organisms (especially coral) which are made of calcium carbonate. Increased solubility of carbonate through increased temperatures results in lower production of marine calcite
Calcite is a Carbonate minerals, carbonate mineral and the most stable Polymorphism (materials science), polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on ...
and increased concentration of atmospheric carbon dioxide. This, in turn, increases Earth temperature. The amount of available is on a geological scale and substantial quantities may eventually be redissolved into the sea and released to the atmosphere, increasing levels even more.
Carbonate salts
* Carbonate overview:Presence outside Earth
It is generally thought that the presence of carbonates inrock
Rock most often refers to:
* Rock (geology), a naturally occurring solid aggregate of minerals or mineraloids
* Rock music, a genre of popular music
Rock or Rocks may also refer to:
Places United Kingdom
* Rock, Caerphilly, a location in Wale ...
is strong evidence for the presence of liquid water. Recent observations of the planetary nebula
A planetary nebula is a type of emission nebula consisting of an expanding, glowing shell of ionized gas ejected from red giant stars late in their lives.
The term "planetary nebula" is a misnomer because they are unrelated to planets. The ...
NGC 6302
NGC 6302 (also known as the Bug Nebula, Butterfly Nebula, or Caldwell 69) is a bipolar planetary nebula in the constellation Scorpius. The structure in the nebula is among the most complex ever seen in planetary nebulae. The spectrum of Butte ...
show evidence for carbonates in space, where aqueous alteration similar to that on Earth is unlikely. Other minerals have been proposed which would fit the observations.
Small amounts of carbonate deposits have been found on Mars via spectral imaging and Martian meteorites also contain small amounts. Groundwater may have existed at Gusev and Meridiani Planum
Meridiani Planum (alternatively Terra Meridiani) is a large plain straddling the equator of Mars. The plain sits on top of an enormous body of sediments that contains bound water. The iron oxide in the spherules is crystalline (grey) hematite (Fe ...
.
See also
* Cap carbonates * Carbon trioxide *Orthocarbonic acid
Orthocarbonic acid, carbon hydroxide, or methanetetrol is the name given to a hypothetical compound with the chemical formula or . Its molecular structure consists of a single carbon atom bonded to four hydroxyl groups. It would be therefore a ...
, , or , a hypothetic unstable molecule
* Oxalate
Oxalate (systematic IUPAC name: ethanedioate) is an anion with the chemical formula . This dianion is colorless. It occurs naturally, including in some foods. It forms a variety of salts, for example sodium oxalate (), and several esters such as ...
* Peroxocarbonate
* Sodium percarbonate
Sodium percarbonate or sodium carbonate peroxide is an inorganic compound with the formula . It is an adduct of sodium carbonate ("soda ash" or "washing soda") and hydrogen peroxide (that is, a perhydrate). It is a colorless, crystalline, hygros ...
References
External links
Carbonate/bicarbonate/carbonic acid equilibrium in water: pH of solutions, buffer capacity, titration and species distribution vs. pH computed with a free spreadsheet
* {{Authority control Carbon oxyanions Oxocarbon anions