Carbon Allotrope on:
[Wikipedia]
[Google]
[Amazon]

 ''Glassy carbon'' or ''vitreous carbon'' is a class of non-graphitizing
''Glassy carbon'' or ''vitreous carbon'' is a class of non-graphitizing
 ''bcc-carbon'': At ultrahigh pressures of above 1000 GPa, diamond is predicted to transform into a
''bcc-carbon'': At ultrahigh pressures of above 1000 GPa, diamond is predicted to transform into a  * The ''
* The ''
 The system of carbon allotropes spans an astounding range of extremes, considering that they are all merely structural formations of the same element.
Between diamond and graphite:
* Diamond crystallizes in the cubic system but graphite crystallizes in the hexagonal system.
* Diamond is clear and transparent, but graphite is black and opaque.
* Diamond is the hardest mineral known (10 on the
The system of carbon allotropes spans an astounding range of extremes, considering that they are all merely structural formations of the same element.
Between diamond and graphite:
* Diamond crystallizes in the cubic system but graphite crystallizes in the hexagonal system.
* Diamond is clear and transparent, but graphite is black and opaque.
* Diamond is the hardest mineral known (10 on the
Carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
is capable of forming many allotropes
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes are different structural modifications of an element: th ...
(structurally different forms of the same element) due to its valency ( tetravalent). Well-known forms of carbon include diamond
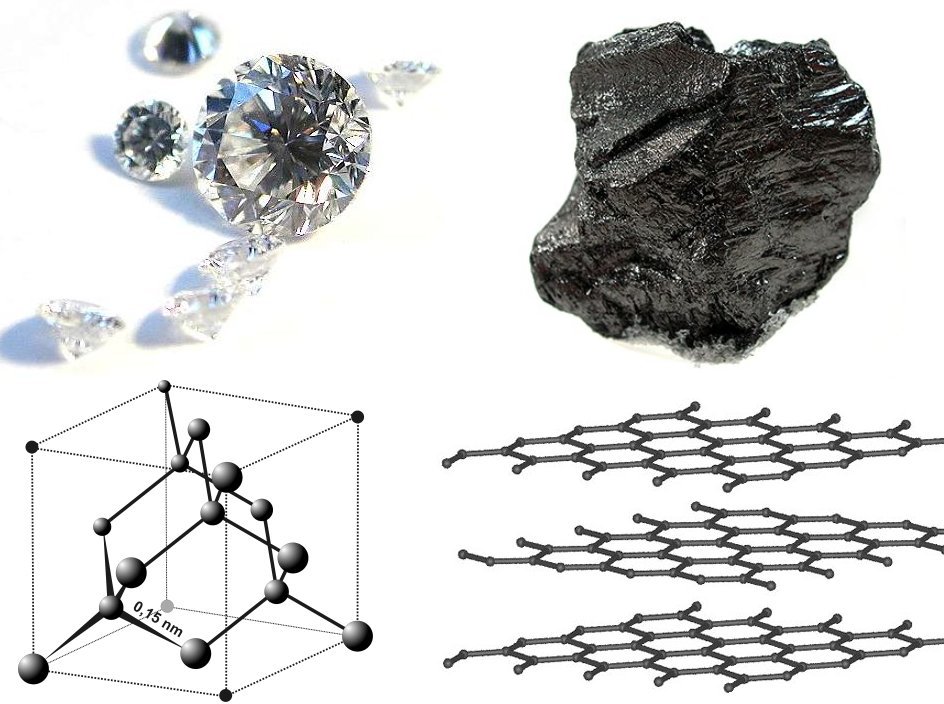
Diamond is a Allotropes of carbon, solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Diamond is tasteless, odourless, strong, brittle solid, colourless in pure form, a poor conductor of e ...
and graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable ...
. In recent decades, many more allotropes have been discovered and researched, including ball shapes such as buckminsterfullerene
Buckminsterfullerene is a type of fullerene with the formula . It has a cage-like fused-ring structure ( truncated icosahedron) made of twenty hexagons and twelve pentagons, and resembles a football. Each of its 60 carbon atoms is bonded to i ...
and sheets such as graphene
Graphene () is a carbon allotrope consisting of a Single-layer materials, single layer of atoms arranged in a hexagonal lattice, honeycomb planar nanostructure. The name "graphene" is derived from "graphite" and the suffix -ene, indicating ...
. Larger-scale structures of carbon include nanotubes, nanobuds and nanoribbons. Other unusual forms of carbon exist at very high temperatures or extreme pressures. Around 500 hypothetical 3‑periodic allotropes of carbon are known at the present time, according to the Samara Carbon Allotrope Database (SACADA).
Atomic and diatomic carbon
Under certain conditions, carbon can be found in its atomic form. It can be formed by vaporizing graphite, by passing large electric currents to form a carbon arc under very low pressure. It is extremely reactive, but it is an intermediate product used in the creation ofcarbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a Valence (chemistry), valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
Th ...
s.
Diatomic carbon
Diatomic carbon (systematically named dicarbon and 1λ2,2λ2-ethene), is a green, gaseous inorganic chemical with the chemical formula C=C (also written 2or C2). It is kinetically unstable at ambient temperature and pressure, being removed throug ...
can also be found under certain conditions. It is often detected via spectroscopy
Spectroscopy is the field of study that measures and interprets electromagnetic spectra. In narrower contexts, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum.
Spectro ...
in extraterrestrial bodies, including comet
A comet is an icy, small Solar System body that warms and begins to release gases when passing close to the Sun, a process called outgassing. This produces an extended, gravitationally unbound atmosphere or Coma (cometary), coma surrounding ...
s and certain star
A star is a luminous spheroid of plasma (physics), plasma held together by Self-gravitation, self-gravity. The List of nearest stars and brown dwarfs, nearest star to Earth is the Sun. Many other stars are visible to the naked eye at night sk ...
s.Diamond
''Diamond'' is a well-known allotrope of carbon. Thehardness
In materials science, hardness (antonym: softness) is a measure of the resistance to plastic deformation, such as an indentation (over an area) or a scratch (linear), induced mechanically either by Pressing (metalworking), pressing or abrasion ...
, extremely high refractive index
In optics, the refractive index (or refraction index) of an optical medium is the ratio of the apparent speed of light in the air or vacuum to the speed in the medium. The refractive index determines how much the path of light is bent, or refrac ...
, and high dispersion of light make diamond useful for industrial applications and for jewelry. Diamond is the hardest known natural mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid substance with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. (2011): Mi ...
. This makes it an excellent abrasive and makes it hold polish and luster extremely well. No known naturally occurring substance can cut or scratch diamond, except another diamond. In diamond form, carbon is one of the costliest elements.
The crystal structure of diamond is a face-centered cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties o ...
lattice having eight atoms per unit cell to form a diamond cubic
In crystallography, the diamond cubic crystal structure is a repeating pattern of 8 atoms that certain materials may adopt as they solidify. While the first known example was diamond, other elements in group 14 also adopt this structure, in ...
structure. Each carbon atom is covalently bonded to four other carbons in a tetrahedral geometry. These tetrahedrons together form a 3-dimensional network of six-membered carbon rings in the chair conformation, allowing for zero bond angle strain. The bonding occurs through sp3 hybridized orbitals to give a C-C bond length
In molecular geometry, bond length or bond distance is defined as the average distance between Atomic nucleus, nuclei of two chemical bond, bonded atoms in a molecule. It is a Transferability (chemistry), transferable property of a bond between at ...
of 154 pm. This network of unstrained covalent bonds makes diamond extremely strong. Diamond is thermodynamically less stable than graphite at pressures below .
The dominant industrial use of diamond is cutting
Cutting is the separation or opening of a physical object, into two or more portions, through the application of an acutely directed force.
Implements commonly used for wikt:cut, cutting are the knife and saw, or in medicine and science the sca ...
, drilling
Drilling is a cutting process where a drill bit is spun to cut a hole of circular cross section (geometry), cross-section in solid materials. The drill bit is usually a rotary Cutting tool (machining), cutting tool, often multi-point. The bit i ...
(drill bit
A drill bit is a cutting tool used in a drill to remove material to create holes, almost always of circular cross-section. Drill bits come in many sizes and shapes and can create different kinds of holes in many different materials. In orde ...
s), grinding (diamond edged cutters), and polishing. Most uses of diamonds in these technologies do not require large diamonds, and most diamonds that are not gem-quality can find an industrial use. Diamonds are embedded in drill tips and saw blades or ground into a powder for use in grinding and polishing applications (due to its extraordinary hardness). Specialized applications include use in laboratories as containment for high pressure experiments (see diamond anvil), high-performance bearings, and specialized window
A window is an opening in a wall, door, roof, or vehicle that allows the exchange of light and may also allow the passage of sound and sometimes air. Modern windows are usually glazed or covered in some other transparent or translucent ma ...
s of technical apparatuses.
The market for industrial-grade diamonds operates much differently from its gem-grade counterpart. Industrial diamonds are valued mostly for their hardness and heat conductivity, making many of the gemological characteristics of diamond, including clarity and color, mostly irrelevant. This helps explain why 80% of mined diamonds (equal to about 100 million carats or 20 tonne
The tonne ( or ; symbol: t) is a unit of mass equal to 1,000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton in the United States to distinguish it from the non-metric units of the s ...
s annually) are unsuitable for use as gemstones and known as ''bort
Bort, boart, or boort is an umbrella term used in the diamond industry to refer to shards of non- gem-grade/quality diamonds. In the manufacturing and heavy industries, "bort" is used to describe dark, imperfectly formed or crystallized diamond ...
'', are destined for industrial use. In addition to mined diamonds, synthetic diamond
A synthetic diamond or laboratory-grown diamond (LGD), also called a lab-grown, laboratory-created, man-made, artisan-created, artificial, or cultured diamond, is a diamond that is produced in a controlled technological process, in contrast to ...
s found industrial applications almost immediately after their invention in the 1950s; another 400 million carats (80 tonnes) of synthetic diamonds are produced annually for industrial use, which is nearly four times the mass of natural diamonds mined over the same period.
With the continuing advances being made in the production of synthetic diamond, future applications are beginning to become feasible. Garnering much excitement is the possible use of diamond as a semiconductor
A semiconductor is a material with electrical conductivity between that of a conductor and an insulator. Its conductivity can be modified by adding impurities (" doping") to its crystal structure. When two regions with different doping level ...
suitable to build microchip
An integrated circuit (IC), also known as a microchip or simply chip, is a set of electronic circuits, consisting of various electronic components (such as transistors, resistors, and capacitors) and their interconnections. These components a ...
s from, or the use of diamond as a heat sink
A heat sink (also commonly spelled heatsink) is a passive heat exchanger that transfers the heat generated by an electronic or a mechanical device to a fluid medium, often air or a liquid coolant, where it is thermal management (electronics), ...
in electronics
Electronics is a scientific and engineering discipline that studies and applies the principles of physics to design, create, and operate devices that manipulate electrons and other Electric charge, electrically charged particles. It is a subfield ...
. Significant research efforts in Japan
Japan is an island country in East Asia. Located in the Pacific Ocean off the northeast coast of the Asia, Asian mainland, it is bordered on the west by the Sea of Japan and extends from the Sea of Okhotsk in the north to the East China Sea ...
, Europe
Europe is a continent located entirely in the Northern Hemisphere and mostly in the Eastern Hemisphere. It is bordered by the Arctic Ocean to the north, the Atlantic Ocean to the west, the Mediterranean Sea to the south, and Asia to the east ...
, and the United States
The United States of America (USA), also known as the United States (U.S.) or America, is a country primarily located in North America. It is a federal republic of 50 U.S. state, states and a federal capital district, Washington, D.C. The 48 ...
are under way to capitalize on the potential offered by diamond's unique material properties, combined with increased quality and quantity of supply starting to become available from synthetic diamond manufacturers.
Graphite
''Graphite'', named byAbraham Gottlob Werner
Abraham Gottlob Werner (; 25 September 174930 June 1817) was a German geologist
A geologist is a scientist who studies the structure, composition, and History of Earth, history of Earth. Geologists incorporate techniques from physics, chem ...
in 1789, from the Greek γράφειν (, "to draw/write", for its use in pencils) is one of the most common allotropes of carbon. Unlike diamond, graphite is an electrical conductor. Thus, it can be used in, for instance, electrical arc lamp electrodes. Likewise, under standard conditions
Standard temperature and pressure (STP) or standard conditions for temperature and pressure are various standard sets of conditions for experimental measurements used to allow comparisons to be made between different sets of data. The most used ...
, graphite is the most stable form of carbon. Therefore, it is used in thermochemistry as the standard state
The standard state of a material (pure substance, mixture or solution) is a reference point used to calculate its properties under different conditions. A degree sign (°) or a superscript ⦵ symbol (⦵) is used to designate a thermodynamic q ...
for defining the heat of formation
In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements in their reference state, wi ...
of carbon compounds.
Graphite conducts electricity, due to delocalization
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref>
The term delocalization is general and can have slightly diff ...
of the pi bond
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbital ...
electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s above and below the planes of the carbon atoms. These electrons are free to move, so are able to conduct electricity. However, the electricity is only conducted along the plane of the layers. In diamond, all four outer electrons of each carbon atom are 'localized' between the atoms in covalent bonding. The movement of electrons is restricted and diamond does not conduct an electric current. In graphite, each carbon atom uses only 3 of its 4 outer energy level electrons in covalently bonding to three other carbon atoms in a plane. Each carbon atom contributes one electron to a delocalized system of electrons that is also a part of the chemical bonding. The delocalized electrons are free to move throughout the plane. For this reason, graphite conducts electricity along the planes of carbon atoms, but does not conduct electricity in a direction at right angles to the plane.
Graphite powder is used as a dry lubricant
A lubricant (sometimes shortened to lube) is a substance that helps to reduce friction between surfaces in mutual contact, which ultimately reduces the heat generated when the surfaces move. It may also have the function of transmitting forces, ...
. Although it might be thought that this industrially important property is due entirely to the loose interlamellar coupling between sheets in the structure, in fact in a vacuum
A vacuum (: vacuums or vacua) is space devoid of matter. The word is derived from the Latin adjective (neuter ) meaning "vacant" or "void". An approximation to such vacuum is a region with a gaseous pressure much less than atmospheric pressur ...
environment (such as in technologies for use in space
Space is a three-dimensional continuum containing positions and directions. In classical physics, physical space is often conceived in three linear dimensions. Modern physicists usually consider it, with time, to be part of a boundless ...
), graphite was found to be a very poor lubricant. This fact led to the discovery that graphite's lubricity is due to adsorbed
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a f ...
air and water between the layers, unlike other layered dry lubricants such as molybdenum disulfide
Molybdenum disulfide (or moly) is an inorganic chemistry, inorganic compound composed of molybdenum and sulfur. Its chemical formula is .
The compound is classified as a transition metal dichalcogenide. It is a silvery black solid that occurs as ...
. Recent studies suggest that an effect called superlubricity
Superlubricity is a regime of relative motion in which friction vanishes or very nearly vanishes. However, the definition of "vanishing" friction level is not clear, which makes the term vague. As an ''ad hoc'' definition, a kinetic coefficient ...
can also account for this effect.
When a large number of crystallographic defects (physical) bind these planes together, graphite loses its lubrication properties and becomes pyrolytic carbon
Pyrolytic carbon is a material similar to graphite, but with some covalent bonding between its graphene sheets as a result of imperfections in its production.
Pyrolytic carbon is man-made and is thought not to be found in nature.Ratner, Buddy D ...
, a useful material in blood-contacting implants such as prosthetic
In medicine, a prosthesis (: prostheses; from ), or a prosthetic implant, is an artificial device that replaces a missing body part, which may be lost through physical trauma, disease, or a condition present at birth (Congenital, congenital disord ...
heart valve
A heart valve is a biological one-way valve that allows blood to flow in one direction through the chambers of the heart. A mammalian heart usually has four valves. Together, the valves determine the direction of blood flow through the heart. Hea ...
s.
Graphite is the most stable allotrope of carbon. Contrary to popular belief, high-purity graphite does not readily burn, even at elevated temperatures. For this reason, it is used in nuclear reactor
A nuclear reactor is a device used to initiate and control a Nuclear fission, fission nuclear chain reaction. They are used for Nuclear power, commercial electricity, nuclear marine propulsion, marine propulsion, Weapons-grade plutonium, weapons ...
s and for high-temperature crucibles for melting metals. At very high temperatures and pressures (roughly 2000 °C and 5 GPa), it can be transformed into diamond.
Natural and crystalline graphites are not often used in pure form as structural materials due to their shear-planes, brittleness and inconsistent mechanical properties.
In its pure glassy (isotropic) synthetic forms, pyrolytic graphite and carbon fiber
Carbon fiber-reinforced polymers (American English), carbon-fibre-reinforced polymers ( Commonwealth English), carbon-fiber-reinforced plastics, carbon-fiber reinforced-thermoplastic (CFRP, CRP, CFRTP), also known as carbon fiber, carbon comp ...
graphite are extremely strong, heat-resistant (to 3000 °C) materials, used in reentry shields for missile nosecones, solid rocket
A solid-propellant rocket or solid rocket is a rocket with a rocket engine that uses solid propellants (fuel/ oxidizer). The earliest rockets were solid-fuel rockets powered by gunpowder. The inception of gunpowder rockets in warfare can be cr ...
engines, high temperature reactors, brake
A brake is a machine, mechanical device that inhibits motion by absorbing energy from a moving system. It is used for Acceleration, slowing or stopping a moving vehicle, wheel, axle, or to prevent its motion, most often accomplished by means of ...
shoes and electric motor
An electric motor is a machine that converts electrical energy into mechanical energy. Most electric motors operate through the interaction between the motor's magnetic field and electric current in a electromagnetic coil, wire winding to gene ...
brushes
A brush is a common tool with bristles, wire or other filaments. It generally consists of a handle or block to which filaments are affixed in either a parallel or perpendicular orientation, depending on the way the brush is to be gripped during u ...
.
Intumescent or expandable graphites are used in fire seals, fitted around the perimeter of a fire door. During a fire the graphite intumesces (expands and chars) to resist fire penetration and prevent the spread of fumes. A typical ''start expansion temperature'' (SET) is between 150 and 300 °C.
Graphite's specific gravity is 2.3, which makes it less dense than diamond.
Graphite is slightly more reactive than diamond. This is because the reactants are able to penetrate between the hexagonal layers of carbon atoms in graphite. It is unaffected by ordinary solvents, dilute acids, or fused alkalis. However, chromic acid
Chromic acid is a chemical compound with the chemical formula . It is also a jargon for a solution formed by the addition of sulfuric acid to aqueous solutions of dichromate. It consists at least in part of chromium trioxide.
The term "chromic ...
oxidizes it to carbon dioxide.
Graphene
A single layer of graphite is calledgraphene
Graphene () is a carbon allotrope consisting of a Single-layer materials, single layer of atoms arranged in a hexagonal lattice, honeycomb planar nanostructure. The name "graphene" is derived from "graphite" and the suffix -ene, indicating ...
and has extraordinary electrical, thermal, and physical properties. It can be produced by epitaxy
Epitaxy (prefix ''epi-'' means "on top of”) is a type of crystal growth or material deposition in which new crystalline layers are formed with one or more well-defined orientations with respect to the crystalline seed layer. The deposited cry ...
on an insulating or conducting substrate or by mechanical exfoliation (repeated peeling) from graphite. Its applications may include replacing silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
in high-performance electronic devices. With two layers stacked, bilayer graphene results with different properties.
Lonsdaleite (hexagonal diamond)
Lonsdaleite
Lonsdaleite (named in honour of Kathleen Lonsdale), also called hexagonal diamond in reference to the crystal structure, is an allotrope of carbon with a hexagonal lattice, as opposed to the cubical lattice of conventional diamond. It is found ...
is an allotrope sometimes called "hexagonal
In geometry, a hexagon (from Greek , , meaning "six", and , , meaning "corner, angle") is a six-sided polygon. The total of the internal angles of any simple (non-self-intersecting) hexagon is 720°.
Regular hexagon
A regular hexagon is d ...
diamond", formed from graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable ...
present in meteor
A meteor, known colloquially as a shooting star, is a glowing streak of a small body (usually meteoroid) going through Earth's atmosphere, after being heated to incandescence by collisions with air molecules in the upper atmosphere,
creating a ...
ites upon their impact on the earth. The great heat and pressure of the impact transforms the graphite into a denser form similar to diamond but retaining graphite's hexagonal crystal lattice
In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystal, crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that ...
. "Hexagonal diamond" has also been synthesized in the laboratory, by compressing and heating graphite either in a static press or using explosives. It can also be produced by the thermal decomposition of a polymer, poly(hydridocarbyne), at atmospheric pressure, under inert gas
An inert gas is a gas that does not readily undergo chemical reactions with other chemical substances and therefore does not readily form chemical compounds. Though inert gases have a variety of applications, they are generally used to prevent u ...
atmosphere (e.g. argon, nitrogen), starting at temperature .
Graphenylene
Graphenylene is a single layer carbon material withbiphenylene
Biphenylene is an organic compound with the formula (C6H4)2. It is a pale, yellowish solid with a hay-like odor. Despite its unusual structure, it behaves like a traditional polycyclic aromatic hydrocarbon.
Bonding
Biphenylene is a polycyclic h ...
-like subunits as basis in its hexagonal lattice structure. It is also known as biphenylene-carbon.
Carbophene
Carbophene is a 2 dimensional covalent organic framework. 4-6 carbophene has been synthesized from 1-3-5 trihydroxybenzene. It consists of 4-carbon and 6-carbon rings in 1:1 ratio. The angles between the three σ-bonds of the orbitals are approximately 120°, 90°, and 150°.AA'-graphite
AA'-graphite is an allotrope of carbon similar to graphite, but where the layers are positioned differently to each other as compared to the order in graphite.Diamane
Diamane is a 2D form of diamond. It can be made via high pressures, but without that pressure, the material reverts to graphene. Another technique is to add hydrogen atoms, but those bonds are weak. Using fluorine (xenon-difluoride) instead brings the layers closer together, strengthening the bonds. This is called f-diamane.Amorphous carbon
''Amorphous carbon'' is the name used forcarbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
that does not have any crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macros ...
line structure. As with all glassy materials, some short-range order can be observed, but there is no long-range pattern of atomic positions. While entirely amorphous carbon can be produced, most amorphous carbon contains microscopic crystals of graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable ...
-like, or even diamond
Diamond is a Allotropes of carbon, solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Diamond is tasteless, odourless, strong, brittle solid, colourless in pure form, a poor conductor of e ...
-like carbon.
Coal
Coal is a combustible black or brownish-black sedimentary rock, formed as rock strata called coal seams. Coal is mostly carbon with variable amounts of other Chemical element, elements, chiefly hydrogen, sulfur, oxygen, and nitrogen.
Coal i ...
and soot
Soot ( ) is a mass of impure carbon particles resulting from the incomplete combustion of hydrocarbons. Soot is considered a hazardous substance with carcinogenic properties. Most broadly, the term includes all the particulate matter produced b ...
or carbon black
Carbon black (with subtypes acetylene black, channel black, furnace black, lamp black and thermal black) is a material produced by the incomplete combustion of coal tar, vegetable matter, or petroleum products, including fuel oil, fluid cataly ...
are informally called amorphous carbon. However, they are products of pyrolysis
Pyrolysis is a process involving the Bond cleavage, separation of covalent bonds in organic matter by thermal decomposition within an Chemically inert, inert environment without oxygen. Etymology
The word ''pyrolysis'' is coined from the Gree ...
(the process of decomposing a substance by the action of heat), which does not produce true amorphous carbon under normal conditions.
Nanocarbons
Buckminsterfullerenes
The ''buckminsterfullerenes'', or usually just ''fullerenes'' or ''buckyballs'' for short, were discovered in 1985 by a team of scientists from Rice University and the University of Sussex, three of whom were awarded the 1996Nobel Prize in Chemistry
The Nobel Prize in Chemistry () is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outst ...
. They are named for the resemblance to the geodesic structures devised by Richard Buckminster "Bucky" Fuller. Fullerenes are positively curved molecules of varying sizes composed entirely of carbon, which take the form of a hollow sphere, ellipsoid, or tube (the C60 version has the same form as a traditional stitched soccer ball).
As of the early twenty-first century, the chemical and physical properties of fullerenes are still under heavy study, in both pure and applied research labs. In April 2003, fullerenes were under study for potential medicinal use — binding specific antibiotics to the structure to target resistant bacteria and even target certain cancer cells such as melanoma.
Nanotubes
Carbon nanotubes, also called buckytubes, are cylindricalcarbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
molecules
A molecule is a group of two or more atoms that are held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemistry ...
with novel properties that make them potentially useful in a wide variety of applications (e.g., nano-electronics, optics
Optics is the branch of physics that studies the behaviour and properties of light, including its interactions with matter and the construction of optical instruments, instruments that use or Photodetector, detect it. Optics usually describes t ...
, materials
A material is a substance or mixture of substances that constitutes an object. Materials can be pure or impure, living or non-living matter. Materials can be classified on the basis of their physical and chemical properties, or on their ge ...
applications, etc.). They exhibit extraordinary strength, unique electrical
Electricity is the set of physical phenomena associated with the presence and motion of matter possessing an electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described by Maxwel ...
properties, and are efficient conductors of heat
In thermodynamics, heat is energy in transfer between a thermodynamic system and its surroundings by such mechanisms as thermal conduction, electromagnetic radiation, and friction, which are microscopic in nature, involving sub-atomic, ato ...
. Non-carbon nanotubes have also been synthesized.
Carbon nanotubes are a members of the fullerene
A fullerene is an allotropes of carbon, allotrope of carbon whose molecules consist of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to six atoms. The molecules may ...
structural family, which also includes buckyballs. Whereas buckyballs are spherical
A sphere (from Ancient Greek, Greek , ) is a surface (mathematics), surface analogous to the circle, a curve. In solid geometry, a sphere is the Locus (mathematics), set of points that are all at the same distance from a given point in three ...
in shape, a nanotube is cylindrical
A cylinder () has traditionally been a Solid geometry, three-dimensional solid, one of the most basic of curvilinear geometric shapes. In elementary geometry, it is considered a Prism (geometry), prism with a circle as its base.
A cylinder may ...
, with at least one end typically capped with a hemisphere of the buckyball structure. Their name is derived from their size, since the diameter of a nanotube is on the order of a few nanometer
330px, Different lengths as in respect to the Molecule">molecular scale.
The nanometre (international spelling as used by the International Bureau of Weights and Measures; SI symbol: nm), or nanometer (American spelling
Despite the va ...
s (approximately 50,000 times smaller than the width of a human hair), while they can be up to several centimeters in length. There are two main types of nanotubes: single-walled nanotubes (SWNTs) and multi-walled nanotubes (MWNTs).
Nanobuds
''Carbon nanobuds'' are a newly discovered allotrope ofcarbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
in which fullerene
A fullerene is an allotropes of carbon, allotrope of carbon whose molecules consist of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to six atoms. The molecules may ...
like "buds" are covalently attached to the outer sidewalls of the carbon nanotubes
A carbon nanotube (CNT) is a tube made of carbon with a diameter in the nanometre range (nanoscale). They are one of the allotropes of carbon. Two broad classes of carbon nanotubes are recognized:
* ''Single-walled carbon nanotubes'' (''SWC ...
. This hybrid material has useful properties of both fullerenes and carbon nanotubes. For instance, they have been found to be exceptionally good field emitters.
Nanolattices
Schwarzites
Schwarzites are negatively curved carbon surfaces originally proposed by decorating triply periodic minimal surfaces with carbon atoms. Thegeometric topology
In mathematics, geometric topology is the study of manifolds and Map (mathematics)#Maps as functions, maps between them, particularly embeddings of one manifold into another.
History
Geometric topology as an area distinct from algebraic topo ...
of the structure is determined by the presence of ring defects, such as heptagons and octagons, to graphene
Graphene () is a carbon allotrope consisting of a Single-layer materials, single layer of atoms arranged in a hexagonal lattice, honeycomb planar nanostructure. The name "graphene" is derived from "graphite" and the suffix -ene, indicating ...
's hexagonal lattice.
(Negative curvature
In mathematics, curvature is any of several strongly related concepts in geometry that intuitively measure the amount by which a curve deviates from being a straight line or by which a surface deviates from being a plane. If a curve or su ...
bends surfaces outwards like a saddle rather than bending inwards like a sphere.)
Recent work has proposed zeolite-templated carbons (ZTCs) may be schwarzites. The name, ZTC, derives from their origin inside the pores of zeolite
Zeolites are a group of several microporous, crystalline aluminosilicate minerals commonly used as commercial adsorbents and catalysts. They mainly consist of silicon, aluminium, oxygen, and have the general formula ・y where is either a meta ...
s, crystalline silicon dioxide
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundan ...
minerals. A vapor of carbon-containing molecules is injected into the zeolite, where the carbon gathers on the pores' walls, creating the negative curve. Dissolving the zeolite leaves the carbon. A team generated structures by decorating the pores of a zeolite with carbon through a Monte Carlo method
Monte Carlo methods, or Monte Carlo experiments, are a broad class of computational algorithms that rely on repeated random sampling to obtain numerical results. The underlying concept is to use randomness to solve problems that might be ...
. Some of the resulting models resemble schwarzite-like structures.
Glassy carbon
 ''Glassy carbon'' or ''vitreous carbon'' is a class of non-graphitizing
''Glassy carbon'' or ''vitreous carbon'' is a class of non-graphitizing carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
widely used as an electrode material in electrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between Electric potential, electrical potential difference and identifiable chemical change. These reactions involve Electron, electrons moving via an electronic ...
, as well as for high-temperature crucibles and as a component of some prosthetic devices.
It was first produced by Bernard Redfern in the mid-1950s at the laboratories of The Carborundum Company, Manchester, UK. He had set out to develop a polymer matrix to mirror a diamond structure and discovered a resole (phenolic) resin that would, with special preparation, set without a catalyst. Using this resin, the first glassy carbon was produced.
The preparation of glassy carbon involves subjecting the organic precursors to a series of heat treatments at temperatures up to 3000 °C. Unlike many non-graphitizing carbons, they are impermeable to gases and are chemically extremely inert, especially those prepared at very high temperatures. It has been demonstrated that the rates of oxidation of certain glassy carbons in oxygen, carbon dioxide or water vapor are lower than those of any other carbon. They are also highly resistant to attack by acids. Thus, while normal graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable ...
is reduced to a powder by a mixture of concentrated sulfuric and nitric acids at room temperature, glassy carbon is unaffected by such treatment, even after several months.
Carbon nanofoam
''Carbon nanofoam'' is the fifth known allotrope of carbon, discovered in 1997 by Andrei V. Rode and co-workers at theAustralian National University
The Australian National University (ANU) is a public university, public research university and member of the Group of Eight (Australian universities), Group of Eight, located in Canberra, the capital of Australia. Its main campus in Acton, A ...
in Canberra
Canberra ( ; ) is the capital city of Australia. Founded following the Federation of Australia, federation of the colonies of Australia as the seat of government for the new nation, it is Australia's list of cities in Australia, largest in ...
. It consists of a low-density cluster-assembly of carbon atoms strung together in a loose three-dimensional web.
Each cluster is about 6 nanometers wide and consists of about 4000 carbon atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
s linked in graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable ...
-like sheets that are given negative curvature by the inclusion of heptagon
In geometry, a heptagon or septagon is a seven-sided polygon or 7-gon.
The heptagon is sometimes referred to as the septagon, using ''Wikt:septa-, septa-'' (an elision of ''Wikt:septua-, septua-''), a Latin-derived numerical prefix, rather than ...
s among the regular hexagon
In geometry, a hexagon (from Greek , , meaning "six", and , , meaning "corner, angle") is a six-sided polygon. The total of the internal angles of any simple (non-self-intersecting) hexagon is 720°.
Regular hexagon
A regular hexagon is de ...
al pattern. This is the opposite of what happens in the case of buckminsterfullerene
Buckminsterfullerene is a type of fullerene with the formula . It has a cage-like fused-ring structure ( truncated icosahedron) made of twenty hexagons and twelve pentagons, and resembles a football. Each of its 60 carbon atoms is bonded to i ...
s, in which carbon sheets are given positive curvature by the inclusion of pentagon
In geometry, a pentagon () is any five-sided polygon or 5-gon. The sum of the internal angles in a simple polygon, simple pentagon is 540°.
A pentagon may be simple or list of self-intersecting polygons, self-intersecting. A self-intersecting ...
s.
The large-scale structure of carbon nanofoam is similar to that of an aerogel
Aerogels are a class of manufacturing, synthetic porous ultralight material derived from a gel, in which the liquid component for the gel has been replaced with a gas, without significant collapse of the gel structure. The result is a solid wit ...
, but with 1% of the density of previously produced carbon aerogels – only a few times the density of air
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosph ...
at sea level
Mean sea level (MSL, often shortened to sea level) is an mean, average surface level of one or more among Earth's coastal Body of water, bodies of water from which heights such as elevation may be measured. The global MSL is a type of vertical ...
. Unlike carbon aerogels, carbon nanofoam is a poor electrical conductor
In physics and electrical engineering, a conductor is an object or type of material that allows the flow of charge (electric current) in one or more directions. Materials made of metal are common electrical conductors. The flow of negatively c ...
.
Carbide-derived carbon
Carbide-derived carbon (CDC) is a family of carbon materials with different surface geometries and carbon ordering that are produced via selective removal of metals from metal carbide precursors, such as TiC, SiC, , , etc. This synthesis is accomplished using chlorine treatment, hydrothermal synthesis, or high-temperature selective metal desorption under vacuum. Depending on the synthesis method, carbide precursor, and reaction parameters, multiple carbon allotropes can be achieved, including endohedral particles composed of predominantly amorphous carbon, carbon nanotubes, epitaxial graphene, nanocrystalline diamond, onion-like carbon, and graphitic ribbons, barrels, and horns. These structures exhibit high porosity and specific surface areas, with highly tunable pore diameters, making them promising materials for supercapacitor-based energy storage, water filtration and capacitive desalinization, catalyst support, and cytokine removal. Other metastable carbon phases, some diamondlike, have been produced from reactions of SiC or CH3SiCl3 with CF4.Linear acetylenic carbon
A one-dimensional carbon polymer with the structure —(C≡C)n—. Its structure is relatively like that of Amorphous carbon.Cyclocarbons
Cyclo 8arbon (C18) was synthesized in 2019.Other possible allotropes
Many other allotropes have been hypothesized but have yet to be synthesized. *body-centered cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the Crystal structure#Unit cell, unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There ...
structure. This phase has importance in astrophysics and deep interiors of planets like Uranus
Uranus is the seventh planet from the Sun. It is a gaseous cyan-coloured ice giant. Most of the planet is made of water, ammonia, and methane in a Supercritical fluid, supercritical phase of matter, which astronomy calls "ice" or Volatile ( ...
and Neptune
Neptune is the eighth and farthest known planet from the Sun. It is the List of Solar System objects by size, fourth-largest planet in the Solar System by diameter, the third-most-massive planet, and the densest giant planet. It is 17 t ...
. Various structures have been proposed. Superdense and superhard material resembling this phase was synthesized and published in 1979 and reported to have the Im space group
In mathematics, physics and chemistry, a space group is the symmetry group of a repeating pattern in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of the pattern that ...
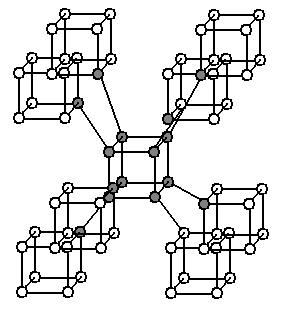
with eight atoms per primitive unit cell (16 atoms per conventional unit cell). Claims were made that the so-called C structure had been synthesized, having eight-carbon cubes similar to cubane
Cubane is a synthetic hydrocarbon compound with the Chemical formula, formula . It consists of eight carbon atoms arranged at the corners of a Cube (geometry), cube, with one hydrogen atom attached to each carbon atom. A solid crystalline substanc ...
in the Imm space group, with eight atoms per primitive unit cell, or 16 atoms per conventional unit cell (also called supercubane, see illustration to the right). But a paper in 1988 claimed that a better theory was that the structure was the same as that of an allotrope of silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
called Si-III or γ-silicon, the so-called BC8 structure with space group Ia and 8 atoms per primitive unit cell (16 atoms per conventional unit cell). In 2008 it was reported that the cubane-like structure had been identified. A paper in 2012 considered four proposed structures, the supercubane structure, the BC8 structure, a structure with clusters of four carbon atoms in tetrahedra in space group I3m having four atoms per primitive unit cell (eight per conventional unit cell), and a structure the authors called "carbon sodalite
Sodalite ( ) is a tectosilicate mineral with the formula , with royal blue varieties widely used as an ornamental gemstone. Although massive sodalite samples are opaque, crystals are usually transparent to translucent. Sodalite is a member of th ...
". They found in favor of this carbon sodalite structure, with a calculated density of 2.927 g/cm, shown in the upper left of the illustration under the abstract. This structure has just six atoms per primitive unit cell (twelve per conventional unit cell). The carbon atoms are in the same locations as the silicon and aluminum atoms of the mineral sodalite. The space group, I3m, is the same as the fully expanded form of sodalite would have if sodalite had just silicon or just aluminum.
* ''bct-carbon'': Body-centered tetragonal carbon was proposed by theorists in 2010.
*'' Chaoite'' is a mineral believed to have been formed in meteorite impacts. It has been described as slightly harder than graphite with a reflection color of grey to white. However, the existence of carbyne phases is disputed – see the article on chaoite for details.
* ''D-carbon'': D-carbon was proposed by theorists in 2018.
D-carbon is an orthorhombic sp3 carbon allotrope (6 atoms per cell). Total-energy calculations demonstrate that D-carbon is energetically more favorable than the previously proposed T6 structure (with 6 atoms per cell) as well as many others.
* Haeckelites: Ordered arrangements of pentagons, hexagons, and heptagons which can either be flat or tubular.
Laves graph
In geometry and crystallography, the Laves graph is an infinite and highly symmetric system of points and line segments in three-dimensional Euclidean space, forming a Periodic graph (geometry), periodic graph. Three equal-length segments meet ...
'' or ''K''4 ''crystal'' is a theoretically predicted three-dimensional crystalline metastable carbon structure in which each carbon atom is bonded to three others, at 120° angles (like graphite), but where the bond planes of adjacent layers lie at an angle of 70.5°, rather than coinciding.
* ''M-carbon'': Monoclinic C-centered carbon is thought to have been first created in 1963 by compressing graphite at room temperature. Its structure was theorized in 2006,
then in 2009 it was related to those experimental observations.
Many structural candidates, including bct-carbon, were proposed to be equally compatible with experimental data available at the time, until in 2012 it was shown theoretically that this structure is kinetically the most likely to form from graphite.
High-resolution data appeared shortly after, demonstrating that among all structure candidates only M-carbon is compatible with experiment.
* ''Metallic carbon'': Theoretical studies have shown that there are regions in the phase diagram, at extremely high pressures, where carbon has metallic character. Laser shock experiments and theory indicate that above 600 GPa liquid carbon is metallic.
*'' Novamene'': A combination of both hexagonal diamond and sp2 hexagons as in graphene.
* '' Phagraphene:'' Graphene-like allotrope with distorted Dirac cones.
* ''Prismane C8'' is a theoretically predicted metastable carbon allotrope
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes are different structural modifications of an element: the ...
comprising an atomic cluster
may refer to:
Science and technology Astronomy
* Cluster (spacecraft), constellation of four European Space Agency spacecraft
* Cluster II (spacecraft), a European Space Agency mission to study the magnetosphere
* Asteroid cluster, a small ...
of eight carbon atoms, with the shape of an elongated triangular bipyramid
In geometry, the elongated triangular bipyramid (or dipyramid) or triakis triangular prism a polyhedron constructed from a triangular prism by attaching two tetrahedrons to its bases. It is an example of Johnson solid.
Construction
The elongate ...
—a six-atom triangular
A triangle is a polygon with three corners and three sides, one of the basic shapes in geometry. The corners, also called ''vertices'', are zero-dimensional points while the sides connecting them, also called ''edges'', are one-dimensional ...
prism
PRISM is a code name for a program under which the United States National Security Agency (NSA) collects internet communications from various U.S. internet companies. The program is also known by the SIGAD . PRISM collects stored internet ...
with two more atoms above and below its bases.
*'' Protomene:'' A hexagonal crystal structure with a fully relaxed primitive cell involving 48 atoms. Out of these, 12 atoms have the potential to switch hybridization between sp2 and sp3, forming dimers.
* '' Q-carbon'': Ferromagnetic
Ferromagnetism is a property of certain materials (such as iron) that results in a significant, observable magnetic permeability, and in many cases, a significant magnetic coercivity, allowing the material to form a permanent magnet. Ferromagne ...
carbon was discovered in 2015.
* ''T-carbon'': Every carbon atom in diamond is replaced with a carbon tetrahedron (hence 'T-carbon'). This was proposed by theorists in 1985.
* There is evidence that white dwarf
A white dwarf is a Compact star, stellar core remnant composed mostly of electron-degenerate matter. A white dwarf is very density, dense: in an Earth sized volume, it packs a mass that is comparable to the Sun. No nuclear fusion takes place i ...
stars have a core of crystallized carbon and oxygen nuclei. The largest of these found in the universe so far, BPM 37093, is located away in the constellation Centaurus
Centaurus () is a bright constellation in the southern sky. One of the 88 modern constellations by area, largest constellations, Centaurus was included among the 48 constellations listed by the 2nd-century astronomer Ptolemy, and it remains one ...
. A news release from the Harvard-Smithsonian Center for Astrophysics described the -wide stellar core as a ''diamond'', and it was named as ''Lucy'', after the Beatles' song "Lucy in the Sky With Diamonds"; however, it is more likely an exotic form of carbon. Penta-graphene is a predicted carbon allotrope that utilizes the Cairo pentagonal tiling.
* ''U carbon'' is predicted to consist of corrugated layers tiled with six- or 12-atom rings, linked by covalent bonds. Notably, it can be harder than steel
Steel is an alloy of iron and carbon that demonstrates improved mechanical properties compared to the pure form of iron. Due to steel's high Young's modulus, elastic modulus, Yield (engineering), yield strength, Fracture, fracture strength a ...
, as conductive as stainless steel, highly reflective and ferromagnetic
Ferromagnetism is a property of certain materials (such as iron) that results in a significant, observable magnetic permeability, and in many cases, a significant magnetic coercivity, allowing the material to form a permanent magnet. Ferromagne ...
, behaving as a permanent magnet
A magnet is a material or object that produces a magnetic field. This magnetic field is invisible but is responsible for the most notable property of a magnet: a force that pulls on other ferromagnetic materials, such as iron, steel, nickel, c ...
at temperatures up to 125 °C.
*'' Zayedene:'' A combination of linear sp carbon chains and sp3 bulk carbon. The structure of these crystalline carbon allotropes consists of sp chains inserted in cylindrical cavities periodically arranged in hexagonal diamond (lonsdaleite).
Variability of carbon
 The system of carbon allotropes spans an astounding range of extremes, considering that they are all merely structural formations of the same element.
Between diamond and graphite:
* Diamond crystallizes in the cubic system but graphite crystallizes in the hexagonal system.
* Diamond is clear and transparent, but graphite is black and opaque.
* Diamond is the hardest mineral known (10 on the
The system of carbon allotropes spans an astounding range of extremes, considering that they are all merely structural formations of the same element.
Between diamond and graphite:
* Diamond crystallizes in the cubic system but graphite crystallizes in the hexagonal system.
* Diamond is clear and transparent, but graphite is black and opaque.
* Diamond is the hardest mineral known (10 on the Mohs scale
The Mohs scale ( ) of mineral hardness is a qualitative ordinal scale, from 1 to 10, characterizing scratch resistance of minerals through the ability of harder material to scratch softer material.
The scale was introduced in 1812 by the Ger ...
), but graphite is one of the softest (1–2 on Mohs scale
The Mohs scale ( ) of mineral hardness is a qualitative ordinal scale, from 1 to 10, characterizing scratch resistance of minerals through the ability of harder material to scratch softer material.
The scale was introduced in 1812 by the Ger ...
).
* Diamond is the ultimate abrasive, but graphite is soft and is a very good lubricant.
* Diamond is an excellent electrical insulator, but graphite is an excellent conductor.
* Diamond is an excellent thermal conductor, but some forms of graphite are used for thermal insulation (for example heat shields and firebreaks).
* At standard temperature and pressure, graphite is the thermodynamically stable form. Thus diamonds do not exist forever. The conversion from diamond to graphite, however, has a very high activation energy
In the Arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur. The activation energy (''E''a) of a reaction is measured in kilojoules per mole (k ...
and is therefore extremely slow.
Despite the hardness of diamonds, the chemical bonds that hold the carbon atoms in diamonds together are actually weaker than those that hold together graphite. The difference is that in diamond, the bonds form an inflexible three-dimensional lattice. In graphite, the atoms are tightly bonded into sheets, but the sheets can slide easily over each other, making graphite soft.
See also
* Superdense carbon allotropesReferences
External links
* * * {{DEFAULTSORT:Carbon Allotropes Carbon