Carbamoyl Chloride on:
[Wikipedia]
[Google]
[Amazon]
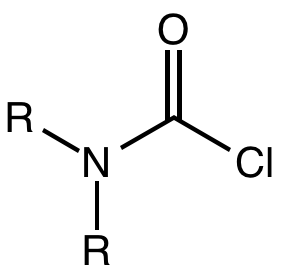 A carbamoyl chloride is the
A carbamoyl chloride is the
 A carbamoyl chloride is the
A carbamoyl chloride is the functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the res ...
with the formula R2NC(O)Cl. The parent carbamoyl chloride, H2NCOCl is unstable, but many N-substituted analogues are known. Most examples are moisture sensitive, colourless, and soluble in nonpolar organic solvents. An example is dimethylcarbamoyl chloride (m.p. −90 °C and b.p. 93 °C). Carbamoyl chlorides are used to prepare a number of pesticides, e.g. carbofuran
Carbofuran is a carbamate pesticide, widely used around the world to control insects on a wide variety of field crops, including potatoes, corn and soybeans. It is a systemic insecticide, which means that the plant absorbs it through the roots ...
and aldicarb
Aldicarb is a carbamate insecticide which is the active substance in the pesticide Temik. It is effective against thrips, aphids, spider mites, lygus, fleahoppers, and leafminers, but is primarily used as a nematicide. Aldicarb is a choli ...
.
Production and examples
Carbamoyl chlorides are prepared by the reaction of an amine with phosgene: :2 R2NH + COCl2 → R2NCOCl + 2NH2l They also arise by the addition ofhydrogen chloride
The compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride g ...
to isocyanate
In organic chemistry, isocyanate is the functional group with the formula . Organic compounds that contain an isocyanate group are referred to as isocyanates. An organic compound with two isocyanate groups is known as a diisocyanate. Diisocyan ...
s:
:RNCO + HCl → RNHCOCl
In this way, carbamonyl chlorides can be prepared with N-H functionality.
Reactions
In a reaction that is typically avoided, hydrolysis of carbamoyl chlorides givescarbamic acid
Carbamic acid, which might also be called aminoformic acid or aminocarboxylic acid, is the chemical compound with the formula . It can be obtained by the reaction of ammonia and carbon dioxide at very low temperatures, which also yields an ...
s:
:R2NCOCl + H2O → R2NC(O)OH + HCl
Owing to the influence of the amino group, these compounds are less hydrolytically sensitive than the usual acid chloride
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group . Their formula is usually written , where R is a side chain. They are reactive derivatives of carboxylic acids (). A specific example o ...
s.
A related but more useful reaction is the analogous reaction with alcohols:{{cite journal, authors=Carol Dallaire, Isabelle Kolber, and Marc Gingras, title=Nickel-Catalyzed Coupling of Aryl O-Carbamates with Grignard Reagents: 2,7-Dimethylnaphthalene, journal=Org. Synth., year=2002, volume=78, pages=42, doi=10.15227/orgsyn.078.0042). Notes: describes use of diethylcarbamoyl chloride as protecting and leaving group.
:R2NCOCl + R'OH + C5H5N → R2NC(O)OR' + C5H5NHCl
References