Capillary Zone Electrophoresis on:
[Wikipedia]
[Google]
[Amazon]
Capillary electrophoresis (CE) is a family of electrokinetic separation methods performed in submillimeter diameter capillaries and in micro- and nanofluidic channels. Very often, CE refers to capillary zone electrophoresis (CZE), but other electrophoretic techniques including capillary
 The instrumentation needed to perform capillary electrophoresis is relatively simple. A basic
The instrumentation needed to perform capillary electrophoresis is relatively simple. A basic
 The velocity of the electroosmotic flow, can be written as:
where is the electroosmotic mobility, which is defined as:
where is the
The velocity of the electroosmotic flow, can be written as:
where is the electroosmotic mobility, which is defined as:
where is the  In certain situations where strong electroosmotic flow toward the cathode is undesirable, the inner surface of the capillary can be coated with polymers, surfactants, or small molecules to reduce electroosmosis to very low levels, restoring the normal direction of migration (anions toward the anode, cations toward the cathode). CE instrumentation typically includes power supplies with reversible polarity, allowing the same instrument to be used in "normal" mode (with EOF and detection near the cathodic end of the capillary) and "reverse" mode (with EOF suppressed or reversed, and detection near the anodic end of the capillary). One of the most common approaches to suppressing EOF, reported by Stellan Hjertén in 1985, is to create a covalently attached layer of linear
In certain situations where strong electroosmotic flow toward the cathode is undesirable, the inner surface of the capillary can be coated with polymers, surfactants, or small molecules to reduce electroosmosis to very low levels, restoring the normal direction of migration (anions toward the anode, cations toward the cathode). CE instrumentation typically includes power supplies with reversible polarity, allowing the same instrument to be used in "normal" mode (with EOF and detection near the cathodic end of the capillary) and "reverse" mode (with EOF suppressed or reversed, and detection near the anodic end of the capillary). One of the most common approaches to suppressing EOF, reported by Stellan Hjertén in 1985, is to create a covalently attached layer of linear
 The resolution () of capillary electrophoresis separations can be written as:
According to this equation,
The resolution () of capillary electrophoresis separations can be written as:
According to this equation,
CE animations
{{Electrophoresis Chromatography Electrophoresis Forensic techniques Polymerase chain reaction
gel electrophoresis
Gel electrophoresis is an electrophoresis method for separation and analysis of biomacromolecules (DNA, RNA, proteins, etc.) and their fragments, based on their size and charge through a gel. It is used in clinical chemistry to separate ...
(CGE), capillary isoelectric focusing (CIEF), capillary isotachophoresis and micellar electrokinetic chromatography (MEKC) belong also to this class of methods. In CE methods, analytes migrate through electrolyte
An electrolyte is a substance that conducts electricity through the movement of ions, but not through the movement of electrons. This includes most soluble Salt (chemistry), salts, acids, and Base (chemistry), bases, dissolved in a polar solven ...
solutions under the influence of an electric field
An electric field (sometimes called E-field) is a field (physics), physical field that surrounds electrically charged particles such as electrons. In classical electromagnetism, the electric field of a single charge (or group of charges) descri ...
. Analytes can be separated according to ionic mobility
Electrical mobility is the ability of charged particles (such as electrons or protons) to move through a medium in response to an electric field that is pulling them. The separation of ions according to their mobility in gas phase is called ion m ...
and/or partitioning into an alternate phase via non-covalent interactions
In chemistry, a non-covalent interaction differs from a covalent bond in that it does not involve the sharing of electrons, but rather involves more dispersed variations of electromagnetic interactions between molecules or within a molecule. The ...
. Additionally, analytes may be concentrated or "focused" by means of gradient
In vector calculus, the gradient of a scalar-valued differentiable function f of several variables is the vector field (or vector-valued function) \nabla f whose value at a point p gives the direction and the rate of fastest increase. The g ...
s in conductivity and pH.
Instrumentation
 The instrumentation needed to perform capillary electrophoresis is relatively simple. A basic
The instrumentation needed to perform capillary electrophoresis is relatively simple. A basic schematic
A schematic, or schematic diagram, is a designed representation of the elements of a system using abstract, graphic symbols rather than realistic pictures. A schematic usually omits all details that are not relevant to the key information the sc ...
of a capillary electrophoresis system is shown in ''figure 1''. The system's main components are a sample vial, source and destination vials, a capillary, electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or a gas). In electrochemical cells, electrodes are essential parts that can consist of a varie ...
s, a high voltage power supply, a detector, and a data output and handling device. The source vial, destination vial and capillary are filled with an electrolyte such as an aqueous buffer solution. To introduce the sample, the capillary inlet is placed into a vial containing the sample. Sample is introduced into the capillary via capillary action
Capillary action (sometimes called capillarity, capillary motion, capillary rise, capillary effect, or wicking) is the process of a liquid flowing in a narrow space without the assistance of external forces like Gravitation, gravity.
The effe ...
, pressure, siphoning, or electrokinetically, and the capillary is then returned to the source vial. The migration of the analytes is initiated by an electric field that is applied between the source and destination vials and is supplied to the electrodes by the high-voltage power supply. In the most common mode of CE, all ions, positive or negative, are pulled through the capillary in the same direction by electroosmotic flow
In chemistry, electro-osmotic flow (EOF, hyphen optional; synonymous with electro-osmosis or electro-endosmosis) is the motion of liquid induced by an applied potential across a porous material, capillary tube, membrane, microchannel, or any oth ...
. The analytes separate as they migrate due to their electrophoretic mobility, and are detected near the outlet end of the capillary. The output of the detector is sent to a data output and handling device such as an integrator
An integrator in measurement and control applications is an element whose output signal is the time integral of its input signal. It accumulates the input quantity over a defined time to produce a representative output.
Integration is an importan ...
or computer
A computer is a machine that can be Computer programming, programmed to automatically Execution (computing), carry out sequences of arithmetic or logical operations (''computation''). Modern digital electronic computers can perform generic set ...
. The data is then displayed as an electropherogram, which reports detector response as a function of time
Time is the continuous progression of existence that occurs in an apparently irreversible process, irreversible succession from the past, through the present, and into the future. It is a component quantity of various measurements used to sequ ...
. Separated chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
s appear as peaks with different migration times in an electropherogram. The technique is often attributed to James W. Jorgensen and Krynn DeArman Lukacs, who first demonstrated the capabilities of this technique. Capillary electrophoresis was first combined with mass spectrometry by Richard D. Smith and coworkers, and provides extremely high sensitivity for the analysis of very small sample sizes. Despite the very small sample sizes (typically only a few nanoliters of liquid are introduced into the capillary), high sensitivity and sharp peaks are achieved in part due to injection strategies that result in a concentration of analytes into a narrow zone near the inlet of the capillary. This is achieved in either pressure or electrokinetic injections simply by suspending the sample in a buffer of lower conductivity (''e.g.'' lower salt concentration) than the running buffer. A process called field-amplified sample stacking (a form of isotachophoresis) results in concentration of analyte in a narrow zone at the boundary between the low-conductivity sample and the higher-conductivity running buffer.
To achieve greater sample throughput, instruments with arrays of capillaries are used to analyze many samples simultaneously. Such capillary array electrophoresis (CAE) instruments with 16 or 96 capillaries are used for medium- to high-throughput capillary DNA sequencing, and the inlet ends of the capillaries are arrayed spatially to accept samples directly from SBS-standard footprint 96-well plates. Certain aspects of the instrumentation (such as detection) are necessarily more complex than for a single-capillary system, but the fundamental principles of design and operation are similar to those shown in Figure 1.
Detection
Separation by capillary electrophoresis can be detected by several detection devices. The majority of commercial systems use UV or UV-Visabsorbance
Absorbance is defined as "the logarithm of the ratio of incident to transmitted radiant power through a sample (excluding the effects on cell walls)". Alternatively, for samples which scatter light, absorbance may be defined as "the negative log ...
as their primary mode of detection. In these systems, a section of the capillary itself is used as the detection cell. The use of on-tube detection enables detection of separated analytes with no loss of resolution. In general, capillaries used in capillary electrophoresis are coated with a polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
(frequently polyimide
Polyimide (sometimes abbreviated PI) is a monomer containing imide groups belonging to the class of high-performance plastics. With their high heat-resistance, polyimides enjoy diverse applications in roles demanding rugged organic materials, suc ...
or Teflon
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene, and has numerous applications because it is chemically inert. The commonly known brand name of PTFE-based composition is Teflon by Chemours, a spin-off from ...
) for increased flexibility. The portion of the capillary used for UV detection, however, must be optically transparent. For polyimide-coated capillaries, a segment of the coating is typically burned or scraped off to provide a bare window several millimeters long. This bare section of capillary can break easily, and capillaries with transparent coatings are available to increase the stability of the cell window. The path length of the detection cell in capillary electrophoresis (~ 50 micrometers
The micrometre (Commonwealth English as used by the International Bureau of Weights and Measures; SI symbol: μm) or micrometer (American English), also commonly known by the non-SI term micron, is a unit of length in the International System ...
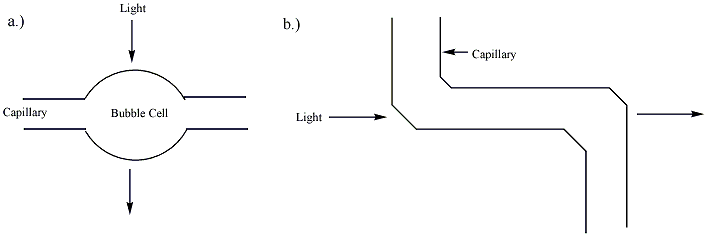
) is far less than that of a traditional UV cell (~ 1 cm). According to the Beer-Lambert law, the sensitivity of the detector is proportional to the path length of the cell. To improve the sensitivity, the path length can be increased, though this results in a loss of resolution. The capillary tube itself can be expanded at the detection point, creating a "bubble cell" with a longer path length or additional tubing can be added at the detection point as shown in ''figure 2''. Both of these methods, however, will decrease the resolution of the separation. This decrease is almost unnoticeable if a smooth aneurysm is produced in the wall of a capillary by heating and pressurization, as plug flow
In fluid mechanics, plug flow is a simple model of the velocity profile of a fluid flowing in a pipe. In plug flow, the velocity of the fluid is assumed to be constant across any cross-section of the pipe perpendicular to the axis of the pipe. ...
can be preserved. This invention by Gary Gordon, US Patent 5061361, typically triples the absorbance path length. When used with a UV absorbance detector, the wider cross-section of the analyte in the cell allows for an illuminating beam twice as large, which reduces shot noise by a factor of two. Together these two factors increase the sensitivity of Agilent Technologies's Bubble Cell CE Detector six times over that of one using a straight capillary. This cell and its manufacture are described on page 62 of the June 1995 issue of the ''Hewlett-Packard Journal''.

Fluorescence
Fluorescence is one of two kinds of photoluminescence, the emission of light by a substance that has absorbed light or other electromagnetic radiation. When exposed to ultraviolet radiation, many substances will glow (fluoresce) with colore ...
detection can also be used in capillary electrophoresis for samples that naturally fluoresce or are chemically modified to contain fluorescent tag
In molecular biology and biotechnology, a fluorescent tag, also known as a fluorescent label or fluorescent probe, is a molecule that is attached chemically to aid in the detection of a biomolecule such as a protein, antibody, or amino acid. Gener ...
s. This mode of detection offers high sensitivity and improved selectivity for these samples, but cannot be utilized for samples that do not fluoresce. Numerous labeling strategies are used to create fluorescent derivatives or conjugates of non-fluorescent molecules, including proteins and DNA. The set-up for fluorescence detection in a capillary electrophoresis system can be complicated. The method requires that the light beam be focused on the capillary, which can be difficult for many light sources. Laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. The word ''laser'' originated as an acronym for light amplification by stimulated emission of radi ...
-induced fluorescence has been used in CE systems with detection limits as low as 10−18 to 10−21 mol. The sensitivity of the technique is attributed to the high intensity
Intensity may refer to:
In colloquial use
* Strength (disambiguation)
*Amplitude
* Level (disambiguation)
* Magnitude (disambiguation)
In physical sciences
Physics
*Intensity (physics), power per unit area (W/m2)
*Field strength of electric, m ...
of the incident light and the ability to accurately focus the light on the capillary. Multi-color fluorescence detection can be achieved by including multiple dichroic mirrors and bandpass filters to separate the fluorescence emission amongst multiple detectors (''e.g.,'' photomultiplier tubes), or by using a prism or grating to project spectrally resolved fluorescence emission onto a position-sensitive detector such as a CCD array. CE systems with 4- and 5-color LIF detection systems are used routinely for capillary DNA sequencing
DNA sequencing is the process of determining the nucleic acid sequence – the order of nucleotides in DNA. It includes any method or technology that is used to determine the order of the four bases: adenine, thymine, cytosine, and guanine. The ...
and genotyping ("DNA fingerprinting
DNA profiling (also called DNA fingerprinting and genetic fingerprinting) is the process of determining an individual's deoxyribonucleic acid (DNA) characteristics. DNA analysis intended to identify a species, rather than an individual, is cal ...
") applications.
In order to obtain the identity of sample components, capillary electrophoresis can be directly coupled with mass spectrometer
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a '' mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is us ...
s or surface-enhanced Raman spectroscopy
Surface-enhanced Raman spectroscopy or surface-enhanced Raman scattering (SERS) is a surface-sensitive technique that enhances Raman scattering by molecules adsorbed on rough metal surfaces or by nanostructures such as plasmonic-magnetic sili ...
(SERS). In most systems, the capillary outlet is introduced into an ion source that utilizes electrospray ionization
Electrospray ionization (ESI) is a technique used in mass spectrometry to produce ions using an electrospray in which a high voltage is applied to a liquid to create an aerosol. It is especially useful in producing ions from macromolecules becau ...
(ESI). The resulting ions are then analyzed by the mass spectrometer. This setup requires volatile buffer solutions, which will affect the range of separation modes that can be employed and the degree of resolution that can be achieved.
The measurement and analysis are mostly done with a specialized.
For CE-SERS, capillary electrophoresis eluants can be deposited onto a SERS-active substrate. Analyte retention times can be translated into spatial distance by moving the SERS-active substrate at a constant rate during capillary electrophoresis. This allows the subsequent spectroscopic technique to be applied to specific eluants for identification with high sensitivity. SERS-active substrates can be chosen that do not interfere with the spectrum of the analytes.
Modes of separation
The separation of compounds by capillary electrophoresis is dependent on the differential migration of analytes in an applied electric field. The electrophoretic migrationvelocity
Velocity is a measurement of speed in a certain direction of motion. It is a fundamental concept in kinematics, the branch of classical mechanics that describes the motion of physical objects. Velocity is a vector (geometry), vector Physical q ...
() of an analyte toward the electrode of opposite charge is:
The electrophoretic mobility can be determined experimentally from the migration time and the field strength:
where is the distance from the inlet to the detection point, is the time required for the analyte to reach the detection point (migration time), is the applied voltage (field strength), and is the total length of the capillary. Since only charged ions are affected by the electric field, neutral analytes are poorly separated by capillary electrophoresis.
The velocity of migration of an analyte in capillary electrophoresis will also depend upon the rate of electroosmotic flow
In chemistry, electro-osmotic flow (EOF, hyphen optional; synonymous with electro-osmosis or electro-endosmosis) is the motion of liquid induced by an applied potential across a porous material, capillary tube, membrane, microchannel, or any oth ...
(EOF) of the buffer solution. In a typical system, the electroosmotic flow is directed toward the negatively charged cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional curren ...
so that the buffer flows through the capillary from the source vial to the destination vial. Separated by differing electrophoretic mobilities, analytes migrate toward the electrode of opposite charge. As a result, negatively charged analytes are attracted to the positively charged anode
An anode usually is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, which is usually an electrode of the device through which conventional current leaves the devic ...
, counter to the EOF, while positively charged analytes are attracted to the cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional curren ...
, in agreement with the EOF as depicted in ''figure 3''.
 The velocity of the electroosmotic flow, can be written as:
where is the electroosmotic mobility, which is defined as:
where is the
The velocity of the electroosmotic flow, can be written as:
where is the electroosmotic mobility, which is defined as:
where is the zeta potential
Zeta potential is the electrical potential at the slipping plane. This plane is the interface which separates mobile fluid from fluid that remains attached to the surface.is a scientific term for Electrokinetic phenomena, electrokinetic Electric ...
of the capillary wall, and is the relative permittivity
The relative permittivity (in older texts, dielectric constant) is the permittivity of a material expressed as a ratio with the vacuum permittivity, electric permittivity of a vacuum. A dielectric is an insulating material, and the dielectric co ...
of the buffer solution. Experimentally, the electroosmotic mobility can be determined by measuring the retention time of a neutral analyte. The velocity () of an analyte in an electric field can then be defined as:
Since the electroosmotic flow of the buffer solution is generally greater than that of the electrophoretic mobility of the analytes, all analytes are carried along with the buffer solution toward the cathode. Even small, triply charged anions can be redirected to the cathode by the relatively powerful EOF of the buffer solution. Negatively charged analytes are retained longer in the capillary due to their conflicting electrophoretic mobilities. The order of migration seen by the detector is shown in ''figure 3'': small multiply charged cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
s migrate quickly and small multiply charged anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
s are retained strongly.
Electroosmotic flow is observed when an electric field is applied to a solution in a capillary that has fixed charges on its interior wall. Charge is accumulated on the inner surface of a capillary when a buffer solution is placed inside the capillary. In a fused-silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant f ...
capillary, silanol
A silanol is a functional group in silicon chemistry with the connectivity Si–O–H. It is related to the hydroxy functional group (C–O–H) found in all alcohols. Silanols are often invoked as intermediates in organosilicon c ...
(Si-OH) groups attached to the interior wall of the capillary are ionized to negatively charged silanoate (Si-O−) groups at pH values greater than three. The ionization of the capillary wall can be enhanced by first running a basic solution, such as NaOH
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula . It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly corrosive base and alkali t ...
or KOH through the capillary prior to introducing the buffer solution. Attracted to the negatively charged silanoate groups, the positively charged cations of the buffer solution will form two inner layers of cations (called the diffuse double layer or the electrical double layer) on the capillary wall as shown in ''figure 4''. The first layer is referred to as the fixed layer because it is held tightly to the silanoate groups. The outer layer, called the mobile layer, is farther from the silanoate groups. The mobile cation layer is pulled in the direction of the negatively charged cathode when an electric field is applied. Since these cations are solvated, the bulk buffer solution migrates with the mobile layer, causing the electroosmotic flow of the buffer solution. Other capillaries including Teflon
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene, and has numerous applications because it is chemically inert. The commonly known brand name of PTFE-based composition is Teflon by Chemours, a spin-off from ...
capillaries also exhibit electroosmotic flow. The EOF of these capillaries is probably the result of adsorption
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a ...
of the electrically charged ions of the buffer onto the capillary walls. The rate of EOF is dependent on the field strength and the charge density of the capillary wall. The wall's charge density is proportional to the pH of the buffer solution. The electroosmotic flow will increase with pH until all of the available silanols lining the wall of the capillary are fully ionized.
polyacrylamide
Polyacrylamide (abbreviated as PAM or pAAM) is a polymer with the formula (-CH2CHCONH2-). It has a linear-chain structure. PAM is highly water-absorbent, forming a soft gel when hydrated. In 2008, an estimated 750,000,000 kg were produced, ...
. The silica surface of the capillary is first modified with a silane reagent bearing a polymerizable vinyl group (''e.g.'' 3-methacryloxypropyltrimethoxysilane), followed by introduction of acrylamide
Acrylamide (or acrylic amide) is an organic compound with the chemical formula CH2=CHC(O)NH2. It is a white odorless solid, soluble in water and several organic solvents. From the chemistry perspective, acrylamide is a vinyl-substituted primary ...
monomer and a free radical initiator. The acrylamide is polymerized ''in situ'', forming long linear chains, some of which are covalently attached to the wall-bound silane reagent. Numerous other strategies for covalent modification of capillary surfaces exist. Dynamic or adsorbed coatings (which can include polymers or small molecules) are also common. For example, in capillary sequencing of DNA, the sieving polymer (typically polydimethylacrylamide) suppresses electroosmotic flow to very low levels. Besides modulating electroosmotic flow, capillary wall coatings can also serve the purpose of reducing interactions between "sticky" analytes (such as proteins) and the capillary wall. Such wall-analyte interactions, if severe, manifest as reduced peak efficiency, asymmetric (tailing) peaks, or even complete loss of analyte to the capillary wall.
Efficiency and resolution
The number of theoretical plates, or separation efficiency, in capillary electrophoresis is given by: where is the number oftheoretical plate
A theoretical plate in many separation processes is a hypothetical zone or stage in which two phases, such as the liquid and vapor phases of a substance, establish an equilibrium with each other. Such equilibrium stages may also be referred to as ...
s, is the apparent mobility in the separation medium and is the diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical p ...
coefficient of the analyte. According to this equation, the efficiency of separation is only limited by diffusion and is proportional to the strength of the electric field, although practical considerations limit the strength of the electric field to several hundred volts per centimeter. Application of very high potentials (>20-30 kV) may lead to arcing or breakdown of the capillary. Further, application of strong electric fields leads to resistive heating (Joule heating) of the buffer in the capillary. At sufficiently high field strengths, this heating is strong enough that radial temperature gradients can develop within the capillary. Since electrophoretic mobility of ions is generally temperature-dependent (due to both temperature-dependent ionization and solvent viscosity effects), a non-uniform temperature profile results in variation of electrophoretic mobility across the capillary, and a loss of resolution. The onset of significant Joule heating can be determined by constructing an "Ohm's Law plot", wherein the current through the capillary is measured as a function of applied potential. At low fields, the current is proportional to the applied potential (Ohm's Law
Ohm's law states that the electric current through a Electrical conductor, conductor between two Node (circuits), points is directly Proportionality (mathematics), proportional to the voltage across the two points. Introducing the constant of ...
), whereas at higher fields the current deviates from the straight line as heating results in decreased resistance of the buffer. The best resolution is typically obtained at the maximum field strength for which Joule heating is insignificant (''i.e.'' near the boundary between the linear and nonlinear regimes of the Ohm's Law plot). Generally capillaries of smaller inner diameter support use of higher field strengths, due to improved heat dissipation and smaller thermal gradients relative to larger capillaries, but with the drawbacks of lower sensitivity in absorbance detection due to shorter path length, and greater difficulty in introducing buffer and sample into the capillary (small capillaries require greater pressure and/or longer times to force fluids through the capillary).
The efficiency of capillary electrophoresis separations is typically much higher than the efficiency of other separation techniques like HPLC
High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify specific components in mixtures. The mixtures can origina ...
. Unlike HPLC, in capillary electrophoresis there is no mass transfer
Mass transfer is the net movement of mass from one location (usually meaning stream, phase, fraction, or component) to another. Mass transfer occurs in many processes, such as absorption, evaporation, drying, precipitation, membrane filtra ...
between phases. In addition, the flow profile in EOF-driven systems is flat, rather than the rounded laminar flow
Laminar flow () is the property of fluid particles in fluid dynamics to follow smooth paths in layers, with each layer moving smoothly past the adjacent layers with little or no mixing. At low velocities, the fluid tends to flow without lateral m ...
profile characteristic of the pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and eve ...
-driven flow in chromatography columns as shown in ''figure 5''. As a result, EOF does not significantly contribute to band broadening as in pressure-driven chromatography. Capillary electrophoresis separations can have several hundred thousand theoretical plates.
 The resolution () of capillary electrophoresis separations can be written as:
According to this equation,
The resolution () of capillary electrophoresis separations can be written as:
According to this equation, maximum
In mathematical analysis, the maximum and minimum of a function (mathematics), function are, respectively, the greatest and least value taken by the function. Known generically as extremum, they may be defined either within a given Interval (ma ...
resolution is reached when the electrophoretic and electroosmotic mobilities are similar in magnitude
Magnitude may refer to:
Mathematics
*Euclidean vector, a quantity defined by both its magnitude and its direction
*Magnitude (mathematics), the relative size of an object
*Norm (mathematics), a term for the size or length of a vector
*Order of ...
and opposite in sign. In addition, it can be seen that high resolution requires lower velocity and, correspondingly, increased analysis time.
Besides diffusion and Joule heating (discussed above), factors that may decrease the resolution in capillary electrophoresis from the theoretical limits in the above equation include, but are not limited to, the finite widths of the injection plug and detection window; interactions between the analyte and the capillary wall; instrumental non-idealities such as a slight difference in height of the fluid reservoirs leading to siphoning; irregularities in the electric field due to, ''e.g.,'' imperfectly cut capillary ends; depletion of buffering capacity in the reservoirs; and electrodispersion (when an analyte has higher conductivity than the background electrolyte). Identifying and minimizing the numerous sources of band broadening is key to successful method development in capillary electrophoresis, with the objective of approaching as close as possible to the ideal of diffusion-limited resolution.
Applications
Capillary electrophoresis may be used for the simultaneous determination of the ions NH4+,, Na+, K+, Mg2+ and Ca2+ insaliva
Saliva (commonly referred as spit or drool) is an extracellular fluid produced and secreted by salivary glands in the mouth. In humans, saliva is around 99% water, plus electrolytes, mucus, white blood cells, epithelial cells (from which ...
.
One of the main applications of CE in forensic science is the development of methods for amplification and detection of DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
fragments using polymerase chain reaction
The polymerase chain reaction (PCR) is a method widely used to make millions to billions of copies of a specific DNA sample rapidly, allowing scientists to amplify a very small sample of DNA (or a part of it) sufficiently to enable detailed st ...
(PCR), which has led to rapid and dramatic advances in forensic DNA analysis. DNA separations are carried out using thin CE 50-mm fused silica capillaries filled with a sieving buffer. These capillaries have excellent capabilities to dissipate heat, permitting much higher electric field strengths to be used than slab gel electrophoresis. Therefore separations in capillaries are rapid and efficient. Additionally, the capillaries can be easily refilled and changed for efficient and automated injections. Detection occurs via fluorescence through a window etched in the capillary. Both single-capillary and capillary-array instruments are available with array systems capable of running 16 or more samples simultaneously for increased throughput.
A major use of CE by forensic biologists is typing of STR from biological samples to generate a profile from highly polymorphic genetic markers which differ between individuals. Other emerging uses for CE include the detection of specific mRNA fragments to help identify the biological fluid or tissue origin of a forensic sample.
Another application of CE in forensics is ink analysis, where the analysis of inkjet printing inks is becoming more necessary due to increasingly frequent counterfeiting of documents printed by inkjet printers. The chemical composition of inks provides very important information in cases of fraudulent documents and counterfeit banknotes. Micellar electrophoretic capillary chromatography (MECC) has been developed and applied to the analysis of inks extracted from paper. Due to its high resolving power relative to inks containing several chemically similar substances, differences between inks from the same manufacturer can also be distinguished. This makes it suitable for evaluating the origin of documents based on the chemical composition of inks. It is worth noting that because of the possible compatibility of the same cartridge with different printer models, the differentiation of inks on the basis of their MECC electrophoretic profiles is a more reliable method for the determination of the ink cartridge of origin (its producer and cartridge number) rather than the printer model of origin.
A specialized type of CE, affinity capillary electrophoresis (ACE), utilizes intermolecular binding interactions to understand protein-ligand interactions. Pharmaceutical companies use ACE for a multitude of reasons, with one of the main ones being the association/binding constants for drugs and ligands or drugs and certain vehicle systems like micelles. It is a widely used technique because of its simplicity, rapid results, and low analyte usage. The use of ACE can provide specific details in binding, separation, and detection of analytes and is proven to be highly practical for studies in life sciences. Aptamer-based affinity capillary electrophoresis is utilized for the analysis and modifications of specific affinity reagents. Modified aptamers ideally exhibit and high binding affinity, specificity, and nuclease resistance. Ren et al. incorporated modified nucleotides in aptamers to introduce new confrontational features and high affinity interactions from the hydrophobic and polar interactions between IL-1α and the aptamer. Huang et al. uses ACE to investigate protein-protein interactions using aptamers. A α-thrombin binding aptamer was labeled with 6-carboxyfluorescein for use as a selective fluorescent probe and was studied to elucidate information on binding sites for protein-protein and protein-DNA interactions.
Capillary electrophoresis (CE) has become an important, cost-effective approach to do DNA sequencing
DNA sequencing is the process of determining the nucleic acid sequence – the order of nucleotides in DNA. It includes any method or technology that is used to determine the order of the four bases: adenine, thymine, cytosine, and guanine. The ...
that provides high throughput and high accuracy sequencing information. Woolley and Mathies used a CE chip to sequence DNA fragments with 97% accuracy and a speed of 150 bases in 540 seconds. They used a 4-color labeling and detection format to collect fluorescent data. Fluorescence is used to view the concentrations of each part of the nucleic acid sequence, A, T, C and G, and these concentration peaks that are graphed from the detection are used to determine the sequence of the DNA.
References
Further reading
* * * * *External links
*CE animations
{{Electrophoresis Chromatography Electrophoresis Forensic techniques Polymerase chain reaction