CYP7A1 on:
[Wikipedia]
[Google]
[Amazon]
Cholesterol 7 alpha-hydroxylase also known as cholesterol 7-alpha-monooxygenase or cytochrome P450 7A1 (CYP7A1) is an

 The increased abundance of SHP causes it to associate with liver receptor homolog (LRH)-1, an obligate factor required for the transcription of CYP7A1. Furthermore, there is an "FXR/SHP-independent" mechanism that also represses CYP7A1 expression. This "FXR/SHP-independent" pathway involves the interaction of bile acids with liver macrophages, which finally induces the expression and secretion of cytokines. These inflammatory cytokines, which include tumor necrosis factor alpha and interleukin-1beta, act upon the liver parenchymal cells causing a rapid repression of the CYP7A1 gene.
The increased abundance of SHP causes it to associate with liver receptor homolog (LRH)-1, an obligate factor required for the transcription of CYP7A1. Furthermore, there is an "FXR/SHP-independent" mechanism that also represses CYP7A1 expression. This "FXR/SHP-independent" pathway involves the interaction of bile acids with liver macrophages, which finally induces the expression and secretion of cytokines. These inflammatory cytokines, which include tumor necrosis factor alpha and interleukin-1beta, act upon the liver parenchymal cells causing a rapid repression of the CYP7A1 gene.
enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecule ...
that in humans is encoded by the gene
In biology, the word gene (from , ; "... Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a b ...
which has an important role in cholesterol metabolism. It is a cytochrome P450
Cytochromes P450 (CYPs) are a superfamily of enzymes containing heme as a cofactor that functions as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are important for the clearance of various compo ...
enzyme, which belongs to the oxidoreductase
In biochemistry, an oxidoreductase is an enzyme that catalyzes the transfer of electrons from one molecule, the reductant, also called the electron donor, to another, the oxidant, also called the electron acceptor. This group of enzymes usually ...
class, and converts cholesterol to 7-alpha-hydroxycholesterol, the first and rate limiting step in bile acid
Bile acids are steroid acids found predominantly in the bile of mammals and other vertebrates. Diverse bile acids are synthesized in the liver. Bile acids are conjugated with taurine or glycine residues to give anions called bile salts.
Primary ...
synthesis.
The inhibition of cholesterol 7-alpha-hydroxylase (CYP7A1) represses bile acid biosynthesis.
Evolution
Sequence comparisons indicated a huge similarity between cytochromes P450 identified in man and bacteria, and suggested that the superfamily cytochrome P450 first originated from a common ancestral gene some three billion years ago. The superfamily cytochrome P450 was named in 1961, because of the 450-nm spectral peak pigment that cytochrome P450 has when reduced and bound to carbon monoxide. In the early 1960s, P450 was thought to be one enzyme, and by the mid 1960s it was associated with drug and steroid metabolism. However, the membrane-associated and hydrophobic nature of the enzyme system impeded purification, and the number of proteins involved could not be accurately counted. Advances in mRNA purification in the early 1980s allowed to isolate the first cDNA encoding a complete cytochrome P450 (CYP) protein, and thereafter, results of many cloning studies have revealed a large number of different enzymes. Advances in molecular biology and genomics facilitated the biochemical characterisation of individual P450 enzymes: * The cytochromes P450 act on many endogenous substrates, introducing oxidative, peroxidative, and reductive changes into small molecules of widely different chemical structures. Substrates identified to date include saturated and unsaturated fatty acids,eicosanoids
Eicosanoids are signaling molecules made by the enzymatic or non-enzymatic oxidation of arachidonic acid or other polyunsaturated fatty acids (PUFAs) that are, similar to arachidonic acid, around 20 carbon units in length. Eicosanoids are a ...
, sterols and steroids, bile acids, vitamin D3 derivatives, retinoids, and uroporphyrinogens Uroporphyrinogens are cyclic tetrapyrroles with four propionic acid groups ("P" groups) and four acetic acid groups ("A" groups).
There are four forms, which vary based upon the arrangements of the "P" and "A" groups (in clockwise order):
* In the ...
.
* Many cytochrome P450 enzymes can metabolise various exogenous compounds including drugs, environmental chemicals and pollutants, and natural plant products.
* Metabolism of foreign chemicals frequently results in successful detoxication of the irritant; However, the actions of P450 enzymes can also generate toxic metabolites that contribute to increased risks of cancer, birth defects, and other toxic effects.
* The expression of many P450 enzymes is often induced by accumulation of a substrate.
* The ability of one P450 substrate to affect the concentrations of another in this manner is the basis for so-called drug-drug interactions, which complicate treatment.
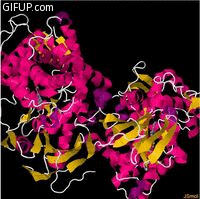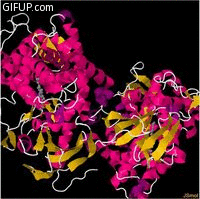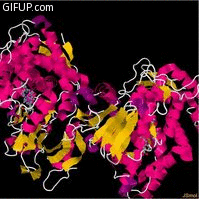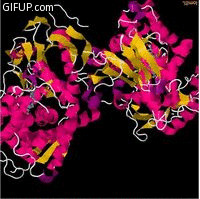
Molecular structure
Cholesterol 7 alpha hydroxylase consists of 491 amino acids, which on folding forms 23alpha helices
The alpha helix (α-helix) is a common motif in the secondary structure of proteins and is a right hand-helix conformation in which every backbone N−H group hydrogen bonds to the backbone C=O group of the amino acid located four residues earli ...
and 26 beta sheet
The beta sheet, (β-sheet) (also β-pleated sheet) is a common motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone hydrogen bonds, forming a gen ...
s.

Function
Cholesterol 7 alpha-hydroxylase is acytochrome P450
Cytochromes P450 (CYPs) are a superfamily of enzymes containing heme as a cofactor that functions as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are important for the clearance of various compo ...
heme
Heme, or haem (pronounced / hi:m/ ), is a precursor to hemoglobin, which is necessary to bind oxygen in the bloodstream. Heme is biosynthesized in both the bone marrow and the liver.
In biochemical terms, heme is a coordination complex "consis ...
enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecule ...
that oxidizes cholesterol in the position 7 using molecular
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as we ...
. It is an oxidoreductase. CYP7A1 is located in the endoplasmic reticulum
The endoplasmic reticulum (ER) is, in essence, the transportation system of the eukaryotic cell, and has many other important functions such as protein folding. It is a type of organelle made up of two subunits – rough endoplasmic reticulum ( ...
(ER) and is important for the synthesis of bile acid and the regulation of cholesterol levels.
Synthesis of bile acid
Cholesterol 7 alpha-hydroxylase is the rate-limiting enzyme in the synthesis ofbile acid
Bile acids are steroid acids found predominantly in the bile of mammals and other vertebrates. Diverse bile acids are synthesized in the liver. Bile acids are conjugated with taurine or glycine residues to give anions called bile salts.
Primary ...
from cholesterol
Cholesterol is any of a class of certain organic molecules called lipids. It is a sterol (or modified steroid), a type of lipid. Cholesterol is biosynthesized by all animal cells and is an essential structural component of animal cell membr ...
via the classic pathway, catalyzing the formation of 7α-hydroxycholesterol. The unique detergent properties of bile acids are essential for the digestion and intestinal absorption of hydrophobic nutrients.
Bile acids have powerful toxic properties like membrane disruption and there are a wide range of mechanisms to restrict their accumulation in tissues and blood. The discovery of farnesoid X receptor
The bile acid receptor (BAR), also known as farnesoid X receptor (FXR) or NR1H4 (nuclear receptor subfamily 1, group H, member 4), is a nuclear receptor that is encoded by the ''NR1H4'' gene in humans.
Function
FXR is expressed at high levels ...
(FXR) which is located in the liver, has opened new insights. Bile acid activation of FXR represses the expression of CYP7A1 via, raising the expression of small heterodimer partner (SHP, NR0B2), a non-DNA binding protein.
 The increased abundance of SHP causes it to associate with liver receptor homolog (LRH)-1, an obligate factor required for the transcription of CYP7A1. Furthermore, there is an "FXR/SHP-independent" mechanism that also represses CYP7A1 expression. This "FXR/SHP-independent" pathway involves the interaction of bile acids with liver macrophages, which finally induces the expression and secretion of cytokines. These inflammatory cytokines, which include tumor necrosis factor alpha and interleukin-1beta, act upon the liver parenchymal cells causing a rapid repression of the CYP7A1 gene.
The increased abundance of SHP causes it to associate with liver receptor homolog (LRH)-1, an obligate factor required for the transcription of CYP7A1. Furthermore, there is an "FXR/SHP-independent" mechanism that also represses CYP7A1 expression. This "FXR/SHP-independent" pathway involves the interaction of bile acids with liver macrophages, which finally induces the expression and secretion of cytokines. These inflammatory cytokines, which include tumor necrosis factor alpha and interleukin-1beta, act upon the liver parenchymal cells causing a rapid repression of the CYP7A1 gene.
Regulation of activity
Regulation of CYP7A1 occurs at several levels including synthesis. Bile acids, steroid hormones, inflammatory cytokines, insulin, and growth factors inhibit CYP7A1 transcription through the 5′-upstream region of the promoter. The average life of this enzyme is between two and three hours. Activity can be regulated by phosphorylation-dephosphorylation. CYP7A1 is upregulated by the nuclear receptor LXR (liver X receptor) when cholesterol (to be specific, oxysterol) levels are high. The effect of this upregulation is to increase the production of bile acids and reduce the level of cholesterol in hepatocytes. It is downregulated by sterol regulatory element-binding proteins (SREBP) when plasma cholesterol levels are low. Bile acids provide feedback inhibition of CYP7A1 by at least two different pathways, both involving the farnesoid X receptor, FXR. In the liver, bile acids bound to FXR induce small heterodimer partner, SHP which binds to LRH-1 and so inhibits the transcription of the enzyme. In the intestine, bile acids/FXR stimulate production of FGF15/19 (depending on species), which then acts as a hormone in the liver via FGFR4.Enzymatic mechanism
Specificity
One feature of enzymes is their high specificity. They are specific on a singular substrate, reaction or both together, that means, that the enzymes can catalyze all reactions wherein the substrate can experience. The enzyme cholesterol 7 alpha hydroxylase catalyzes the reaction that converts cholesterol into cholesterol 7 alpha hydroxylase reducing and oxidizing that molecule.Interactive pathway map
Clinical significance
Deficiency of this enzyme will increase the possibility of cholesterol gallstones. Disruption of CYP7A1 from classic bile acid synthesis in mice leads to either increased postnatal death or a milder phenotype with elevated serum cholesterol. The latter is similar to the case in humans, where CYP7A1 mutations associate with high plasma low-density lipoprotein and hepatic cholesterol content, as well as deficient bile acid excretion. There is also a synergy between plasma low-density lipoprotein cholesterol (LDL-C) and risks ofcoronary artery disease
Coronary artery disease (CAD), also called coronary heart disease (CHD), ischemic heart disease (IHD), myocardial ischemia, or simply heart disease, involves Ischemia, the reduction of blood flow to the myocardium, heart muscle due to build-up o ...
(CAD). Glucose signaling also induces CYP7A1 gene transcription by epigenetic regulation of the histone acetylation
Histone acetyltransferases (HATs) are enzymes that acetylate conserved lysine amino acids on histone proteins by transferring an acetyl group from acetyl-CoA to form ε-''N''-acetyllysine. DNA is wrapped around histones, and, by transferring an a ...
status. Glucose induction of bile acid synthesis have an important implication in metabolic control of glucose, lipid, and energy homeostasis under normal and diabetic conditions. CYP7A1-rs3808607 and apolipoprotein E
Apolipoprotein E (APOE) is a protein involved in the metabolism of fats in the body of mammals. A subtype is implicated in Alzheimer's disease and cardiovascular disease.
APOE belongs to a family of fat-binding proteins called apolipoproteins. ...
(APOE) isoform are associated with the extent of reduction in circulating LDL cholesterol in response to plant sterol consumption and could serve as potential predictive genetic markers to identify individuals who would derive maximum LDL cholesterol lowering with plant sterol consumption. Genetic variations in CYP7A1 influence its expression and thus may affect the risk of gallstone disease and gallbladder cancer.
One of the many lipid lowering effects of the fibrate drug class is mediated through the inhibition of transcription of this enzyme. This inhibition leads to more cholesterol in the bile, which is the body's only route of cholesterol excretion. This also increases the risk of cholesterol gallstone formation.
Inhibition of CYP7A1 is thought to be involved in or responsible for the hepatotoxicity
Hepatotoxicity (from ''hepatic toxicity'') implies chemical-driven liver damage. Drug-induced liver injury is a cause of acute and chronic liver disease caused specifically by medications and the most common reason for a drug to be withdrawn fr ...
associated with ketoconazole
Ketoconazole, sold under the brand name Nizoral among others, is an antiandrogen and antifungal medication used to treat a number of fungal infections. Applied to the skin it is used for fungal skin infections such as tinea, cutaneous can ...
. The levorotatory enantiomer
Optical rotation, also known as polarization rotation or circular birefringence, is the rotation of the orientation of the plane of polarization about the optical axis of linearly polarized light as it travels through certain materials. Circula ...
of ketoconazole, levoketoconazole, shows 12-fold reduced potency in inhibition of this enzyme, and is under development for certain indications (e.g., Cushing's syndrome
Cushing's syndrome is a collection of signs and symptoms due to prolonged exposure to glucocorticoids such as cortisol. Signs and symptoms may include high blood pressure, abdominal obesity but with thin arms and legs, reddish stretch marks ...
) as a replacement for ketoconazole with reduced toxicity
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a subs ...
and improved tolerability and safety
Safety is the state of being "safe", the condition of being protected from harm or other danger. Safety can also refer to the control of recognized hazards in order to achieve an acceptable level of risk.
Meanings
There are two slightly di ...
.
See also
* Steroidogenic enzymeReferences
Further reading
* * * * * * * * * * * * * * * * * * * *External links
* * {{Enzymes EC 1.14.13 Metabolism Enzymes of known structure