But-2-ene-hydration-2D-skeletal on:
[Wikipedia]
[Google]
[Amazon]
In
Blue Book
. Ideally, every possible organic
Different side-chains and functional groups will be grouped together in alphabetical order. (The multiplier prefixes di-, tri-, etc. are not taken into consideration for grouping alphabetically. For example, ethyl comes before dihydroxy or dimethyl, as the "e" in "ethyl" precedes the "h" in "dihydroxy" and the "m" in "dimethyl" alphabetically. The "di" is not considered in either case). When both side chains and secondary functional groups are present, they should be written mixed together in one group rather than in two separate groups. # Identification of double/triple bonds. # Numbering of the chain. This is done by first numbering the chain in both directions (left to right and right to left), and then choosing the numbering which follows these rules, in order of precedence. Not every rule will apply to every compound, rules can be skipped if they do not apply. ## Has the lowest-numbered
Wherever it says "with numbers", it is understood that between the word and the numbers, the prefix (di-, tri-) is used. # Adding of punctuation: ## Commas are put between numbers (2 5 5 becomes 2,5,5) ## Hyphens are put between a number and a letter (2 5 5 trimethylheptane becomes 2,5,5-trimethylheptane) ## Successive words are merged into one word (trimethyl heptane becomes trimethylheptane)
Note: IUPAC uses one-word names throughout. This is why all parts are connected. The resulting name appears as: :
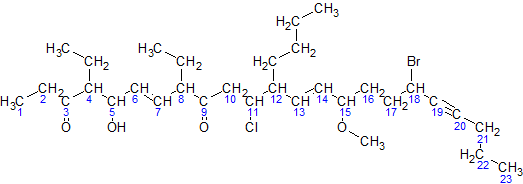 For simplicity, here is an image of the same molecule, where the hydrogens in the parent chain are removed and the carbons are shown by their numbers:
For simplicity, here is an image of the same molecule, where the hydrogens in the parent chain are removed and the carbons are shown by their numbers:
 Now, following the above steps:
# The parent
Now, following the above steps:
# The parent
Note: the at carbon atom 15 is not a side chain, but it is a methoxy functional group. #* There are two ethyl- groups. They are combined to create, 4,8-diethyl. #* The side chains are grouped like this: 12-butyl-4,8-diethyl. (But this is not necessarily the final grouping, as functional groups may be added in between to ensure all groups are listed alphabetically.) # The secondary functional groups are: a hydroxy- at carbon 5, a chloro- at carbon 11, a methoxy- at carbon 15, and a bromo- at carbon 18. Grouped with the side chains, this gives 18-bromo-12-butyl-11-chloro-4,8-diethyl-5-hydroxy-15-methoxy. # There are two double bonds: one between carbons 6 and 7, and one between carbons 13 and 14. They would be called "6,13-diene", but the presence of alkynes switches it to 6,13-dien. There is one triple bond between carbon atoms 19 and 20. It will be called 19-yne. # The arrangement (with punctuation) is: 18-bromo-12-butyl-11-chloro-4,8-diethyl-5-hydroxy-15-methoxytricosa-6,13-dien-19-yne-3,9-dione # Finally, due to cis-trans isomerism, we have to specify the relative orientation of functional groups around each double bond. For this example, both double bonds are trans isomers, so we have (6''E'',13''E'') The final name is (6''E'',13''E'')-18-bromo-12-butyl-11-chloro-4,8-diethyl-5-hydroxy-15-methoxytricosa-6,13-dien-19-yne-3,9-dione.

 Branched alkanes are named as a straight-chain alkane with attached
Branched alkanes are named as a straight-chain alkane with attached  If there are multiple side-branches of the same size alkyl group, their positions are separated by commas and the group prefixed with multiplier prefixes depending on the number of branches. For example, (neopentane) is named 2,2-dimethylpropane. If there are different groups, they are added in alphabetical order, separated by commas or hyphens. The longest possible main alkane chain is used; therefore 3-ethyl-4-methylhexane instead of 2,3-diethylpentane, even though these describe equivalent structures. The di-, tri- etc. prefixes are ignored for the purpose of alphabetical ordering of
If there are multiple side-branches of the same size alkyl group, their positions are separated by commas and the group prefixed with multiplier prefixes depending on the number of branches. For example, (neopentane) is named 2,2-dimethylpropane. If there are different groups, they are added in alphabetical order, separated by commas or hyphens. The longest possible main alkane chain is used; therefore 3-ethyl-4-methylhexane instead of 2,3-diethylpentane, even though these describe equivalent structures. The di-, tri- etc. prefixes are ignored for the purpose of alphabetical ordering of 

 Alkenes are named for their parent alkane chain with the suffix "
Alkenes are named for their parent alkane chain with the suffix "
 Alkynes are named using the same system, with the suffix "
Alkynes are named using the same system, with the suffix "
 In haloalkanes and haloarenes (),
In haloalkanes and haloarenes (),
 Alcohols () take the suffix " -ol" with a numerical suffix indicating the bonding position: is propan-1-ol. The suffixes , , , etc., are used for multiple groups:
Alcohols () take the suffix " -ol" with a numerical suffix indicating the bonding position: is propan-1-ol. The suffixes , , , etc., are used for multiple groups:  If higher precedence functional groups are present (see ''
If higher precedence functional groups are present (see ''
 Ethers () consist of an oxygen atom between the two attached carbon chains. The shorter of the two chains becomes the first part of the name with the -ane suffix changed to -oxy, and the longer alkane chain becomes the suffix of the name of the ether. Thus, is methoxymethane, and is
Ethers () consist of an oxygen atom between the two attached carbon chains. The shorter of the two chains becomes the first part of the name with the -ane suffix changed to -oxy, and the longer alkane chain becomes the suffix of the name of the ether. Thus, is methoxymethane, and is
 Aldehydes () take the suffix " -al". If other functional groups are present, the chain is numbered such that the aldehyde carbon is in the "1" position, unless functional groups of higher precedence are present.
If a prefix form is required, "oxo-" is used (as for ketones), with the position number indicating the end of a chain: is 3-oxopropanoic acid. If the carbon in the carbonyl group cannot be included in the attached chain (for instance in the case of cyclic aldehydes), the prefix "formyl-" or the suffix "-carbaldehyde" is used: is cyclohexanecarbaldehyde. If an aldehyde is attached to a benzene and is the main functional group, the suffix becomes benzaldehyde.
Aldehydes () take the suffix " -al". If other functional groups are present, the chain is numbered such that the aldehyde carbon is in the "1" position, unless functional groups of higher precedence are present.
If a prefix form is required, "oxo-" is used (as for ketones), with the position number indicating the end of a chain: is 3-oxopropanoic acid. If the carbon in the carbonyl group cannot be included in the attached chain (for instance in the case of cyclic aldehydes), the prefix "formyl-" or the suffix "-carbaldehyde" is used: is cyclohexanecarbaldehyde. If an aldehyde is attached to a benzene and is the main functional group, the suffix becomes benzaldehyde.
 In general ketones () take the suffix " -one" (pronounced ''own'', not ''won'') with a suffixed position number: is pentan-2-one. If a higher precedence suffix is in use, the prefix "oxo-" is used: is 3-oxohexanal.
In general ketones () take the suffix " -one" (pronounced ''own'', not ''won'') with a suffixed position number: is pentan-2-one. If a higher precedence suffix is in use, the prefix "oxo-" is used: is 3-oxohexanal.
 In general, carboxylic acids () are named with the suffix '' -oic acid'' (etymologically a
In general, carboxylic acids () are named with the suffix '' -oic acid'' (etymologically a

 Esters () are named as alkyl derivatives of carboxylic acids. The alkyl (R') group is named first. The part is then named as a separate word based on the carboxylic acid name, with the ending changed from "-oic acid" to " -oate" or "-carboxylate" For example, is methyl pentanoate, and is ethyl 4-methylpentanoate. For esters such as
Esters () are named as alkyl derivatives of carboxylic acids. The alkyl (R') group is named first. The part is then named as a separate word based on the carboxylic acid name, with the ending changed from "-oic acid" to " -oate" or "-carboxylate" For example, is methyl pentanoate, and is ethyl 4-methylpentanoate. For esters such as
retained names
The "-oate" changes to "-ate." Some simple examples, named both ways, are shown in the figure above. If the alkyl group is not attached at the end of the chain, the bond position to the ester group is suffixed before "-yl": may be called butan-2-yl propanoate or butan-2-yl propionate.. The prefix form is "oxycarbonyl-" with the (R') group preceding.
If the alkyl group is not attached at the end of the chain, the bond position to the ester group is suffixed before "-yl": may be called butan-2-yl propanoate or butan-2-yl propionate.. The prefix form is "oxycarbonyl-" with the (R') group preceding.
 Acyl groups are named by stripping the "-ic acid" of the corresponding carboxylic acid and replacing it with "-yl." For example, is called ethanoyl-R.
Acyl groups are named by stripping the "-ic acid" of the corresponding carboxylic acid and replacing it with "-yl." For example, is called ethanoyl-R.
 Simply add the name of the attached halide to the end of the acyl group. For example, is ethanoyl chloride. An alternate suffix is "-carbonyl halide" as opposed to "-oyl halide". The prefix form is "halocarbonyl-".
Simply add the name of the attached halide to the end of the acyl group. For example, is ethanoyl chloride. An alternate suffix is "-carbonyl halide" as opposed to "-oyl halide". The prefix form is "halocarbonyl-".

 Amines () are named for the attached alkane chain with the suffix "-amine" (e.g., methanamine). If necessary, the bonding position is suffixed: propan-1-amine, propan-2-amine. The prefix form is "amino-".
For secondary amines (of the form ), the longest carbon chain attached to the nitrogen atom becomes the primary name of the amine; the other chain is prefixed as an alkyl group with location prefix given as an italic ''N'': is ''N''-methylethanamine. Tertiary amines () are treated similarly: is ''N''-ethyl-''N''-methylpropanamine. Again, the substituent groups are ordered alphabetically.
Amines () are named for the attached alkane chain with the suffix "-amine" (e.g., methanamine). If necessary, the bonding position is suffixed: propan-1-amine, propan-2-amine. The prefix form is "amino-".
For secondary amines (of the form ), the longest carbon chain attached to the nitrogen atom becomes the primary name of the amine; the other chain is prefixed as an alkyl group with location prefix given as an italic ''N'': is ''N''-methylethanamine. Tertiary amines () are treated similarly: is ''N''-ethyl-''N''-methylpropanamine. Again, the substituent groups are ordered alphabetically.
 Amides () take the suffix "-amide", or "-carboxamide" if the carbon in the amide group cannot be included in the main chain. The prefix form is "carbamoyl-". e.g., methanamide, ethanamide.
Amides that have additional substituents on the nitrogen are treated similarly to the case of amines: they are ordered alphabetically with the location prefix ''N'': is ''N'',''N''-dimethylmethanamide, is ''N'',''N''-dimethylethanamide.
Amides () take the suffix "-amide", or "-carboxamide" if the carbon in the amide group cannot be included in the main chain. The prefix form is "carbamoyl-". e.g., methanamide, ethanamide.
Amides that have additional substituents on the nitrogen are treated similarly to the case of amines: they are ordered alphabetically with the location prefix ''N'': is ''N'',''N''-dimethylmethanamide, is ''N'',''N''-dimethylethanamide.
 Nitriles () are named by adding the suffix "-nitrile" to the longest hydrocarbon chain (including the carbon of the cyano group). It can also be named by replacing the "-oic acid" of their corresponding carboxylic acids with "-carbonitrile." The prefix form is "cyano-." Functional class IUPAC nomenclature may also be used in the form of alkyl cyanides. For example, is called pentanenitrile or butyl cyanide.
Nitriles () are named by adding the suffix "-nitrile" to the longest hydrocarbon chain (including the carbon of the cyano group). It can also be named by replacing the "-oic acid" of their corresponding carboxylic acids with "-carbonitrile." The prefix form is "cyano-." Functional class IUPAC nomenclature may also be used in the form of alkyl cyanides. For example, is called pentanenitrile or butyl cyanide.

acceptable IUPAC names
IUPAC Nomenclature of Organic Chemistry
(online version of several older editions of the
IUPAC Recommendations on Organic & Biochemical Nomenclature, Symbols, Terminology, etc.
(includes IUBMB Recommendations for biochemistry)
(last updated 11 April 2003)
ACD/Name
Software for generating systematic nomenclature
ChemAxon Name <> Structure
– ChemAxon IUPAC (& traditional) name to structure and structure to IUPAC name software. As used a
chemicalize.orgchemicalize.org
A free web site/service that extracts IUPAC names from web pages and annotates a 'chemicalized' version with structure images. Structures from annotated pages can also be searched. *
* {{Organic chemistry Chemical nomenclature Encodings Organic chemistry
chemical nomenclature
Chemical nomenclature is a set of rules to generate systematic name#In chemistry, systematic names for chemical compounds. The nomenclature used most frequently worldwide is the one created and developed by the International Union of Pure and Appli ...
, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compound
Some chemical authorities define an organic compound as a chemical compound that contains a Carbon–hydrogen bond, carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. F ...
s as recommended by the International Union of Pure and Applied Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
(IUPAC). It is published in the ''Nomenclature of Organic Chemistry
''Nomenclature of Organic Chemistry'', commonly referred to by chemists as the ''Blue Book'', is a collection of recommendations on organic chemical nomenclature published at irregular intervals by the International Union of Pure and Applied C ...
'' (informally called thBlue Book
. Ideally, every possible organic
compound
Compound may refer to:
Architecture and built environments
* Compound (enclosure), a cluster of buildings having a shared purpose, usually inside a fence or wall
** Compound (fortification), a version of the above fortified with defensive struc ...
should have a name from which an unambiguous structural formula
The structural formula of a chemical compound is a graphic representation of the molecular structure (determined by structural chemistry methods), showing how the atoms are connected to one another. The chemical bonding within the molecule is al ...
can be created. There is also an IUPAC nomenclature of inorganic chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
.
To avoid long and tedious names in normal communication, the official IUPAC naming recommendations are not always followed in practice, except when it is necessary to give an unambiguous and absolute definition to a compound. IUPAC names can sometimes be simpler than older names, as with ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the ps ...
, instead of ethyl alcohol. For relatively simple molecules they can be more easily understood than non-systematic names, which must be learnt or looked over. However, the common or trivial name
In chemistry, a trivial name is a non-systematic name for a chemical substance. That is, the name is not recognized according to the rules of any formal system of chemical nomenclature such as IUPAC inorganic or IUPAC organic nomenclature. A ...
is often substantially shorter and clearer, and so preferred. These non-systematic names are often derived from an original source of the compound. Also, very long names may be less clear than structural formulas.
Basic principles
In chemistry, a number ofprefix
A prefix is an affix which is placed before the stem of a word. Particularly in the study of languages, a prefix is also called a preformative, because it alters the form of the word to which it is affixed.
Prefixes, like other affixes, can b ...
es, suffixes
In linguistics, a suffix is an affix which is placed after the stem of a word. Common examples are case endings, which indicate the grammatical case of nouns and adjectives, and verb endings, which form the conjugation of verbs. Suffixes can ca ...
and infix
An infix is an affix inserted inside a word stem (an existing word or the core of a family of words). It contrasts with '' adfix,'' a rare term for an affix attached to the outside of a stem, such as a prefix or suffix.
When marking text for ...
es are used to describe the type and position of the functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
s in the compound.
The steps for naming an organic compound are:
# Identification of the most senior group. If more than one functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
, if any, is present, the one with highest group precedence should be used.
# Identification of the ring or chain with the maximum number of senior groups.
# Identification of the ring or chain with the most senior elements (In order: N, P, Si, B, O, S, C).
# Identification of the parent compound. Rings are senior to chains if composed of the same elements.
## For cyclic systems: Identification of the parent cyclic ring. The cyclic system must obey these rules, in order of precedence:
### It should have the most senior heteroatom
In chemistry, a heteroatom () is, strictly, any atom that is not carbon or hydrogen.
Organic chemistry
In practice, the term is mainly used more specifically to indicate that non-carbon atoms have replaced carbon in the backbone of the molecular ...
(in order: N, O, S, P, Si, B).
### It should have the maximum number of rings.
### It should have the maximum number of atoms.
### It should have the maximum number of heteroatoms.
### It should have the maximum number of senior heteroatoms (in order: O, S, N, P, Si, B).
## For chains: Identification of the parent hydrocarbon chain. This chain must obey the following rules, in order of precedence:
### It should have the maximum length.
### It should have the maximum number of heteroatoms.
### It should have the maximum number of senior heteroatoms (in order: O, S, N, P, Si, B).
##For cyclic systems and chains after previous rules:
###It should have the maximum number of multiple, then double bonds.
###It should have the maximum number of substituents of the suffix functional group. By suffix, it is meant that the parent functional group should have a suffix, unlike halogen substituents. If more than one functional group is present, the one with highest group precedence should be used.
# Identification of the side-chains. Side chains are the carbon chains that are not in the parent chain, but are branched off from it.
# Identification of the remaining functional groups, if any, and naming them by their ionic prefixes (such as hydroxy for , oxy for , oxyalkane for , etc.).Different side-chains and functional groups will be grouped together in alphabetical order. (The multiplier prefixes di-, tri-, etc. are not taken into consideration for grouping alphabetically. For example, ethyl comes before dihydroxy or dimethyl, as the "e" in "ethyl" precedes the "h" in "dihydroxy" and the "m" in "dimethyl" alphabetically. The "di" is not considered in either case). When both side chains and secondary functional groups are present, they should be written mixed together in one group rather than in two separate groups. # Identification of double/triple bonds. # Numbering of the chain. This is done by first numbering the chain in both directions (left to right and right to left), and then choosing the numbering which follows these rules, in order of precedence. Not every rule will apply to every compound, rules can be skipped if they do not apply. ## Has the lowest-numbered
locant
In the nomenclature of organic chemistry, a locant is a term to indicate the position of a functional group or substituent within a molecule.
Numeric locants
The International Union of Pure and Applied Chemistry (IUPAC) recommends the use of n ...
(or locants) for heteroatoms. Locants are the numbers on the carbons to which the substituent is directly attached.
## Has the lowest-numbered locants for the indicated hydrogen. The indicated hydrogen is for some unsaturated heterocyclic compounds. It refers to the hydrogen atoms not attached to atoms with double bonds in the ring system.
## Has the lowest-numbered locants for the suffix functional group.
## Has the lowest-numbered locants for multiple bonds ('ene', 'yne'), and hydro prefixes. (The locant of a multiple bond is the number of the adjacent carbon with a lower number).
## Has the lowest-numbered locants for all substituents cited by prefixes.
## Has the lowest-numbered locants for substituents in order of citation (for example: in a cyclic ring with only bromine and chlorine functional groups, alphabetically bromo- is cited before chloro- and would receive the lower locant).
# Numbering of the various substituents and bonds with their locants. If there is more than one of the same type of substituent/double bond, a prefix is added showing how many there are (di – 2, tri – 3, tetra – 4, then as for the number of carbons below with 'a' added at the end)
The numbers for that type of side chain will be grouped in ascending order and written before the name of the side-chain. If there are two side-chains with the same alpha carbon
In the nomenclature of organic chemistry, a locant is a term to indicate the position of a functional group or substituent within a molecule.
Numeric locants
The International Union of Pure and Applied Chemistry (IUPAC) recommends the use of n ...
, the number will be written twice. Example: 2,2,3-trimethyl- . If there are both double bonds and triple bonds, "en" (double bond) is written before "yne" (triple bond). When the main functional group is a terminal functional group (a group which can exist only at the end of a chain, like formyl and carboxyl groups), there is no need to number it.
# Arrangement in this form: Group of side chains and secondary functional groups with numbers made in step 6 + prefix of parent hydrocarbon chain (eth, meth) + double/triple bonds with numbers (or "ane") + primary functional group suffix with numbers.Wherever it says "with numbers", it is understood that between the word and the numbers, the prefix (di-, tri-) is used. # Adding of punctuation: ## Commas are put between numbers (2 5 5 becomes 2,5,5) ## Hyphens are put between a number and a letter (2 5 5 trimethylheptane becomes 2,5,5-trimethylheptane) ## Successive words are merged into one word (trimethyl heptane becomes trimethylheptane)
Note: IUPAC uses one-word names throughout. This is why all parts are connected. The resulting name appears as: :
Example
Here is a sample molecule with the parent carbons numbered: For simplicity, here is an image of the same molecule, where the hydrogens in the parent chain are removed and the carbons are shown by their numbers:
For simplicity, here is an image of the same molecule, where the hydrogens in the parent chain are removed and the carbons are shown by their numbers:
 Now, following the above steps:
# The parent
Now, following the above steps:
# The parent hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually fain ...
chain has 23 carbons. It is called tricosa-.
# The functional groups with the highest precedence are the two ketone groups.
## The groups are on carbon atoms 3 and 9. As there are two, we write 3,9-dione.
## The numbering of the molecule is based on the ketone groups. When numbering from left to right, the ketone groups are numbered 3 and 9. When numbering from right to left, the ketone groups are numbered 15 and 21. 3 is less than 15, therefore the ketones are numbered 3 and 9. The smaller number is always used, not the sum of the constituents numbers.
# The side chains are: an ethyl- at carbon 4, an ethyl- at carbon 8, and a butyl- at carbon 12. Note: the at carbon atom 15 is not a side chain, but it is a methoxy functional group. #* There are two ethyl- groups. They are combined to create, 4,8-diethyl. #* The side chains are grouped like this: 12-butyl-4,8-diethyl. (But this is not necessarily the final grouping, as functional groups may be added in between to ensure all groups are listed alphabetically.) # The secondary functional groups are: a hydroxy- at carbon 5, a chloro- at carbon 11, a methoxy- at carbon 15, and a bromo- at carbon 18. Grouped with the side chains, this gives 18-bromo-12-butyl-11-chloro-4,8-diethyl-5-hydroxy-15-methoxy. # There are two double bonds: one between carbons 6 and 7, and one between carbons 13 and 14. They would be called "6,13-diene", but the presence of alkynes switches it to 6,13-dien. There is one triple bond between carbon atoms 19 and 20. It will be called 19-yne. # The arrangement (with punctuation) is: 18-bromo-12-butyl-11-chloro-4,8-diethyl-5-hydroxy-15-methoxytricosa-6,13-dien-19-yne-3,9-dione # Finally, due to cis-trans isomerism, we have to specify the relative orientation of functional groups around each double bond. For this example, both double bonds are trans isomers, so we have (6''E'',13''E'') The final name is (6''E'',13''E'')-18-bromo-12-butyl-11-chloro-4,8-diethyl-5-hydroxy-15-methoxytricosa-6,13-dien-19-yne-3,9-dione.
Hydrocarbons
Alkanes
Straight-chain
In chemistry, an open-chain compound (or open chain compound) or acyclic compound (Greek prefix ''α'' 'without' and ''κύκλος'' 'cycle') is a compound with a linear structure, rather than a cyclic one.
An open-chain compound having no side ...
alkanes take the suffix "-ane
In organic chemistry, the suffix ''-ane'' forms the names of organic compounds where the group (a carbon-carbon single bond) has been attributed the highest priority according to the rules of organic nomenclature. Such organic compounds are call ...
" and are prefixed depending on the number of carbon atoms in the chain, following standard rules. The first few are:
For example, the simplest alkane is methane, and the nine-carbon alkane is named nonane
Nonane is a linear alkane hydrocarbon with the chemical formula C9H20. It is a colorless, flammable liquid, occurring primarily in the component of the petroleum distillate fraction commonly called kerosene, which is used as a heating, tractor, a ...
. The names of the first four alkanes were derived from methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often ab ...
, ether
In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group (e.g., alkyl or aryl). They have the general formula , where R and R� ...
, propionic acid
Propionic acid (, from the Greek language, Greek words πρῶτος : ''prōtos'', meaning "first", and πίων : ''píōn'', meaning "fat"; also known as propanoic acid) is a naturally occurring carboxylic acid with chemical formula . It is a ...
and butyric acid
Butyric acid (; from , meaning "butter"), also known under the systematic name butanoic acid, is a straight-chain alkyl carboxylic acid with the chemical formula . It is an oily, colorless liquid with an unpleasant odor. Isobutyric acid (2-met ...
, respectively. The rest are named with a Greek
Greek may refer to:
Anything of, from, or related to Greece, a country in Southern Europe:
*Greeks, an ethnic group
*Greek language, a branch of the Indo-European language family
**Proto-Greek language, the assumed last common ancestor of all kno ...
numeric prefix, with the exceptions of nonane which has a Latin
Latin ( or ) is a classical language belonging to the Italic languages, Italic branch of the Indo-European languages. Latin was originally spoken by the Latins (Italic tribe), Latins in Latium (now known as Lazio), the lower Tiber area aroun ...
prefix, and undecane
Undecane (also known as hendecane) is a liquid alkane hydrocarbon with the chemical formula CH3(CH2)9CH3. It is used as a mild sex attractant for various types of moths and cockroaches, and an alert signal for a variety of ants. It has 159 isomer ...
which has mixed-language prefixes.
Cyclic alkanes are simply prefixed with "cyclo-": for example, is cyclobutane (not to be confused with butene
Butene, also known as butylene, is an alkene with the formula . The word ''butene'' may refer to any of the individual compounds. They are colourless gases that are present in crude oil as a minor constituent in quantities that are too small for ...
) and is cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colourless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexan ...
(not to be confused with hexene
In organic chemistry, hexene is a hydrocarbon with the chemical formula . The prefix "hex" is derived from the fact that there are 6 carbon atoms in the molecule, while the "-ene" suffix denotes that there is an alkene present—two carbon atoms ...
).
alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl group is derived from a cy ...
groups. They are prefixed with a number indicating the carbon the group is attached to, counting from the end of the alkane chain. For example, , commonly known as isobutane
Isobutane, also known as ''i''-butane, 2-methylpropane or methylpropane, is a chemical compound with molecular formula HC(CH3)3. It is an isomer of butane. Isobutane is a colorless, odorless gas.
It is the simplest alkane with a tertiary carbon a ...
, is treated as a propane chain with a methyl group bonded to the middle (2) carbon, and given the systematic name 2-methylpropane. However, although the name 2-methylpropane ''could'' be used, it is easier and more logical to call it simply methylpropane – the methyl group could not possibly occur on any of the other carbon atoms (that would lengthen the chain and result in butane, not propane) and therefore the use of the number "2" is unnecessary.
If there is ambiguity in the position of the substituent, depending on which end of the alkane chain is counted as "1", then numbering is chosen so that the smaller number is used. For example, (isopentane) is named 2-methylbutane, not 3-methylbutane.
side chain
In organic chemistry and biochemistry, a side chain is a substituent, chemical group that is attached to a core part of the molecule called the "main chain" or backbone chain, backbone. The side chain is a hydrocarbon branching element of a mo ...
s (e.g. 3-ethyl-2,4-dimethylpentane, not 2,4-dimethyl-3-ethylpentane).
Alkenes
-ene
The suffix -ene is used in organic chemistry to form names of organic compounds where the -C=C- group has been attributed the highest priority according to the rules of organic nomenclature. Sometimes a number between hyphens is inserted before ...
" and a numerical root indicating the position of the carbon with the lower number for each double bond in the chain: is but-1-ene.
Multiple double bonds take the form -diene, -triene, etc., with the size prefix of the chain taking an extra "a": is buta-1,3-diene. Simple cis and trans CIS may refer to:
Computing
* Card information structure, formatting and organization data stored on a PC card
* Center for Internet Security, cybersecurity benchmarks, controls, practices and tools
* Center for Internet and Society (disambiguati ...
isomers
In chemistry, isomers are molecules or polyatomic ions with identical molecular formula – that is, the same number of atoms of each element – but distinct arrangements of atoms in space. ''Isomerism'' refers to the existence or possibili ...
may be indicated with a prefixed ''cis-'' or ''trans-'': ''cis''-but-2-ene, ''trans''-but-2-ene. However, ''cis-'' and ''trans-'' are ''relative'' descriptors. It is IUPAC convention to describe all alkenes using ''absolute'' descriptors of ''Z-'' (same side) and ''E-'' (opposite) with the Cahn–Ingold–Prelog priority rules
In organic chemistry, the Cahn–Ingold–Prelog (CIP) sequence rules (also the CIP priority convention; named after Robert Sidney Cahn, Christopher Kelk Ingold, and Vladimir Prelog) are a standard process to completely and unequivocally nam ...
(see also E–Z notation
''E''–''Z'' configuration, or the ''E''–''Z'' convention, is the IUPAC preferred method of describing the absolute stereochemistry of double bonds in organic chemistry. It is an extension of ''cis''–''trans'' isomer notation (which o ...
).
Alkynes
-yne
In chemistry, the suffix ''-yne'' is used to denote the presence of a triple bond ().
The suffix follows IUPAC nomenclature, and is mainly used in organic chemistry. However, inorganic compounds featuring unsaturation in the form of triple bo ...
" indicating a triple bond: ethyne (acetylene
Acetylene (Chemical nomenclature, systematic name: ethyne) is a chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is u ...
), propyne (methylacetylene
Propyne (methylacetylene) is an alkyne with the chemical formula . It is a component of MAPD gas—along with its isomer propadiene (allene), which was commonly used in gas welding. Unlike acetylene, propyne can be safely condensed.Peter Päs ...
).
Functional groups
Haloalkanes and haloarenes
Halogen
The halogens () are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would ...
functional groups are prefixed with the bonding position and take the form of fluoro-, chloro-, bromo-, iodo-, etc., depending on the halogen. Multiple groups are dichloro-, trichloro-, etc., and dissimilar groups are ordered alphabetically as before. For example, (chloroform
Chloroform, or trichloromethane (often abbreviated as TCM), is an organochloride with the formula and a common solvent. It is a volatile, colorless, sweet-smelling, dense liquid produced on a large scale as a precursor to refrigerants and po ...
) is trichloromethane. The anesthetic halothane
Halothane, sold under the brand name Fluothane among others, is a general anaesthetic. It can be used to induce or maintain anaesthesia. One of its benefits is that it does not increase the production of saliva, which can be particularly useful ...
() is 2-bromo-2-chloro-1,1,1-trifluoroethane.
Alcohols
Ethylene glycol
Ethylene glycol ( IUPAC name: ethane-1,2-diol) is an organic compound (a vicinal diol) with the formula . It is mainly used for two purposes: as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an odo ...
is ethane-1,2-diol.
order of precedence
An order of precedence is a sequential hierarchy of importance applied to individuals, groups, or organizations. For individuals, it is most often used for diplomats in attendance at very formal occasions. It can also be used in the context of ...
'', below), the prefix "hydroxy" is used with the bonding position: is 2-hydroxypropanoic acid.
Ethers
methoxyethane
Methoxyethane, also known as ethyl methyl ether, is a colorless gaseous ether with the formula . Unlike the related dimethyl ether and diethyl ether, which are widely used and studied, this mixed alkyl ether has no current applications. It is a st ...
(''not'' ethoxymethane). If the oxygen is not attached to the end of the main alkane chain, then the whole shorter alkyl-plus-ether group is treated as a side-chain and prefixed with its bonding position on the main chain. Thus is 2-methoxypropane.
Alternatively, an ether chain can be named as an alkane in which one carbon is replaced by an oxygen, a replacement denoted by the prefix "oxa". For example, could also be called 2-oxabutane, and an epoxide
In organic chemistry, an epoxide is a cyclic ether, where the ether forms a three-atom ring: two atoms of carbon and one atom of oxygen. This triangular structure has substantial ring strain, making epoxides highly reactive, more so than other ...
could be called oxacyclopropane. This method is especially useful when both groups attached to the oxygen atom are complex.
Aldehydes
Ketones
Carboxylic acids
back-formation
Back-formation is the process or result of creating a neologism, new word via Morphology (linguistics), morphology, typically by removing or substituting actual or supposed affixes from a lexical item, in a way that expands the number of lexemes ...
from benzoic acid
Benzoic acid () is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. The benzoyl group is often abbreviated "Bz" (not to be confused with "Bn," which ...
). As with aldehydes, the carboxyl
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl group (e.g. ...
functional group must take the "1" position on the main chain and so the locant need not be stated. For example, (lactic acid
Lactic acid is an organic acid. It has the molecular formula C3H6O3. It is white in the solid state and it is miscible with water. When in the dissolved state, it forms a colorless solution. Production includes both artificial synthesis as wel ...
) is named 2-hydroxypropanoic acid with no "1" stated. Some traditional names for common carboxylic acids (such as acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
) are in such widespread use that they are retained
In the United Kingdom and Ireland, a retained firefighter, also known as an RDS firefighter or on-call firefighter, is a firefighter who does not work on a fire station full-time but is paid to spend long periods of time on call to respond to eme ...
in IUPAC nomenclature, though systematic names like ethanoic acid are also used. Carboxylic acids attached to a benzene ring are structural analog
A structural analog, also known as a chemical analog or simply an analog, is a chemical compound, compound having a chemical structure, structure similar to that of another compound, but differing from it in respect to a certain component.
It can ...
s of benzoic acid () and are named as one of its derivatives.
If there are multiple carboxyl groups on the same parent chain, multiplying prefixes are used: Malonic acid
Malonic acid is a dicarboxylic acid with structure CH2(COOH)2. The ionized form of malonic acid, as well as its esters and salts, are known as malonates. For example, diethyl malonate is malonic acid's diethyl ester. The name originates from ...
, , is systematically named propanedioic acid. Alternatively, the suffix can be used in place of "oic acid", combined with a multiplying prefix if necessary – mellitic acid
Mellitic acid, also called graphitic acid or benzenehexacarboxylic acid, is an acid first discovered in 1799 by Martin Heinrich Klaproth in the mineral mellite (honeystone), which is the aluminium salt of the acid. It crystallizes in fine silky n ...
is benzenehexacarboxylic acid, for example. In the latter case, the carbon atoms in the carboxyl groups do ''not'' count as being part of the main chain, a rule that also applies to the prefix form "carboxy-". Citric acid
Citric acid is an organic compound with the formula . It is a Transparency and translucency, colorless Weak acid, weak organic acid. It occurs naturally in Citrus, citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, ...
serves as an example: it is formally named rather than .
Carboxylates
Salts
In chemistry, a salt or ionic compound is a chemical compound consisting of an assembly of positively charged ions ( cations) and negatively charged ions (anions), which results in a compound with no net electric charge (electrically neutral). ...
of carboxylic acids are named following the usual cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
-then-anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
conventions used for ionic compounds
In chemistry, a salt or ionic compound is a chemical compound consisting of an assembly of positively charged ions ( cations) and negatively charged ions (anions), which results in a compound with no net electric charge (electrically neutral). ...
in both IUPAC and common nomenclature systems. The name of the carboxylate anion () is derived from that of the parent acid by replacing the "–oic acid" ending with "–oate" or "carboxylate." For example, , the sodium salt of benzoic acid
Benzoic acid () is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. The benzoyl group is often abbreviated "Bz" (not to be confused with "Bn," which ...
(), is called sodium benzoate. Where an acid has both a systematic and a common name (like , for example, which is known as both acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
and as ethanoic acid), its salts can be named from either parent name. Thus, can be named as potassium acetate
Potassium acetate (also called potassium ethanoate), (CH3COOK) is the potassium salt of acetic acid. It is a hygroscopic solid at room temperature.
Preparation
It can be prepared by treating a potassium-containing base such as potassium hydroxide ...
or as potassium ethanoate. The prefix form, is "carboxylato-".
Esters
ethyl acetate
Ethyl acetate commonly abbreviated EtOAc, ETAC or EA) is the organic compound with the formula , simplified to . This flammable, colorless liquid has a characteristic sweet smell (similar to pear drops) and is used in glues, nail polish removers, ...
(), ethyl formate
Ethyl formate is an ester formed when ethanol (an alcohol) reacts with formic acid (a carboxylic acid). Ethyl formate has the characteristic smell of rum and is partially responsible for the flavor of raspberries, occurring naturally in some plan ...
() or dimethyl phthalate that are based on common acids, IUPAC recommends use of these established names, calleretained names
The "-oate" changes to "-ate." Some simple examples, named both ways, are shown in the figure above.
Acyl groups
Acyl halides
Acid anhydrides
Acid anhydrides () have two acyl groups linked by an oxygen atom. If both acyl groups are the same, then the name of the carboxylic acid with the word acid is replaced with the word ''anhydride'' and the IUPAC name consists of two words. If the acyl groups are different, then they are named in alphabetical order in the same way, with ''anhydride'' replacing ''acid'' and IUPAC name consists of three words. For example, is called ''ethanoic anhydride'' and is called ''ethanoic propanoic anhydride''.Amines
Amides
Nitriles
 Nitriles () are named by adding the suffix "-nitrile" to the longest hydrocarbon chain (including the carbon of the cyano group). It can also be named by replacing the "-oic acid" of their corresponding carboxylic acids with "-carbonitrile." The prefix form is "cyano-." Functional class IUPAC nomenclature may also be used in the form of alkyl cyanides. For example, is called pentanenitrile or butyl cyanide.
Nitriles () are named by adding the suffix "-nitrile" to the longest hydrocarbon chain (including the carbon of the cyano group). It can also be named by replacing the "-oic acid" of their corresponding carboxylic acids with "-carbonitrile." The prefix form is "cyano-." Functional class IUPAC nomenclature may also be used in the form of alkyl cyanides. For example, is called pentanenitrile or butyl cyanide.
Cyclic compounds
Cycloalkane
In organic chemistry, the cycloalkanes (also called naphthenes, but distinct from naphthalene) are the ring (chemistry), monocyclic Saturated and unsaturated compounds, saturated hydrocarbons. In other words, a cycloalkane consists only of hydroge ...
s and aromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugati ...
compounds can be treated as the main parent chain of the compound, in which case the positions of substituents are numbered around the ring structure. For example, the three isomers of xylene
In organic chemistry, xylene or xylol (; IUPAC name: dimethylbenzene) are any of three organic compounds with the formula . They are derived from the substitution of two hydrogen atoms with methyl groups in a benzene ring; which hydrogens are su ...
, commonly the ''ortho-'', ''meta-
''Meta'' (from the , '' meta'', meaning 'after' or 'beyond') is an adjective meaning 'more comprehensive' or 'transcending'.
In modern nomenclature, the prefix
meta can also serve as a prefix meaning self-referential, as a field of study or endea ...
'', and ''para-'' forms, are 1,2-dimethylbenzene, 1,3-dimethylbenzene, and 1,4-dimethylbenzene. The cyclic structures can also be treated as functional groups themselves, in which case they take the prefix "cyclo''alkyl''-" (e.g. "cyclohexyl-") or for benzene, "phenyl-".
The IUPAC nomenclature scheme becomes rapidly more elaborate for more complex cyclic structures, with notation for compounds containing conjoined rings, and many common names such as phenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire.
The molecule consists of a phenyl group () ...
being accepted as base names for compounds derived from them.
Order of precedence of group
When compounds contain more than one functional group, the order of precedence determines which groups are named with prefix or suffix forms. The table below shows common groups in decreasing order of precedence. The highest-precedence group takes the suffix, with all others taking the prefix form. However, double and triple bonds only take suffix form (-en and -yn) and are used with other suffixes. Prefixed substituents are ordered alphabetically (excluding any modifiers such as di-, tri-, etc.), e.g. chlorofluoromethane, ''not'' fluorochloromethane. If there are multiple functional groups of the same type, either prefixed or suffixed, the position numbers are ordered numerically (thus ethane-1,2-diol, ''not'' ethane-2,1-diol.) The ''N'' position indicator for amines and amides comes before "1", e.g., is ''N'',2-dimethylpropanamine. *''Note'': These suffixes, in which the carbon atom is counted as part of the preceding chain, are the most commonly used. See individual functional group articles for more details. The order of remaining functional groups is only needed for substituted benzene and hence is not mentioned here.Common nomenclature – trivial names
Common nomenclature uses the older names for some organic compounds instead of using the prefixes for the carbon skeleton above. The pattern can be seen below.Ketones
Common names forketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
s can be derived by naming the two alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl group is derived from a cy ...
or aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
groups bonded to the carbonyl group
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such as aldehydes ...
as separate words followed by the word ''ketone''.
*Acetone
Acetone (2-propanone or dimethyl ketone) is an organic compound with the chemical formula, formula . It is the simplest and smallest ketone (). It is a colorless, highly Volatile organic compound, volatile, and flammable liquid with a charact ...
*Acetophenone
Acetophenone is the organic compound with the formula C6H5C(O)CH3. It is the simplest aromatic ketone. This colorless, viscous liquid is a precursor to useful resins and fragrances.
Production
Acetophenone is formed as a byproduct of the cumene ...
*Benzophenone
Benzophenone is a naturally occurring organic compound with the formula (C6H5)2CO, generally abbreviated Ph2CO. Benzophenone has been found in some fungi, fruits and plants, including grapes. It is a white solid with a low melting point and ros ...
* Ethyl isopropyl ketone
* Diethyl ketone
The first three of the names shown above are still considered to bacceptable IUPAC names
Aldehydes
The common name for analdehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
is derived from the common name of the corresponding carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
by dropping the word ''acid'' and changing the suffix from -ic or -oic to -aldehyde.
*Formaldehyde
Formaldehyde ( , ) (systematic name methanal) is an organic compound with the chemical formula and structure , more precisely . The compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde. It is stored as ...
*Acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic compound, organic chemical compound with the chemical formula, formula , sometimes abbreviated as . It is a colorless liquid or gas, boiling near room temperature. It is one of the most ...
Ions
The IUPAC nomenclature also provides rules for namingion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
s.
Hydron
Hydron
In chemistry, the hydron, informally called proton, is the cationic form of atomic hydrogen, represented with the symbol . The general term "hydron", endorsed by IUPAC, encompasses cations of hydrogen regardless of isotope: thus it refers collec ...
is a generic term for hydrogen cation; protons, deuterons and tritons are all hydrons.
The hydrons are not found in heavier isotopes, however.
Parent hydride cations
Simple cations formed by adding ahydron
In chemistry, the hydron, informally called proton, is the cationic form of atomic hydrogen, represented with the symbol . The general term "hydron", endorsed by IUPAC, encompasses cations of hydrogen regardless of isotope: thus it refers collec ...
to a hydride of a halogen, chalcogen
The chalcogens (ore forming) ( ) are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family. Group 16 consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the rad ...
or pnictogen
, -
! colspan=2 style="text-align:left;" , ↓ Period
, -
! 2
,
, -
! 3
,
, -
! 4
,
, -
! 5
,
, -
! 6
,
, -
! 7
,
, -
, colspan="2",
----
''Legend''
A pnictogen ( or ; from "to choke" and -gen, "generator") is any ...
are named by adding the suffix "-onium" to the element's root: is ammonium, is oxonium, and H2F+ is fluoronium. Ammonium was adopted instead of nitronium, which commonly refers to .
If the cationic center of the hydride is not a halogen, chalcogen or pnictogen then the suffix "-ium" is added to the name of the neutral hydride after dropping any final 'e'. is methanium, is dioxidanium (HO-OH is dioxidane), and is diazanium ( is diazane).
Cations and substitution
The above cations except for methanium are not, strictly speaking, organic, since they do not contain carbon. However, many organic cations are obtained by substituting another element or some functional group for a hydrogen. The name of each substitution is prefixed to the hydride cation name. If many substitutions by the same functional group occur, then the number is indicated by prefixing with "di-", "tri-" as with halogenation. is trimethyloxonium. is trifluoromethylammonium.See also
* Descriptor (chemistry) *Hantzsch–Widman nomenclature
In organic chemistry, Hantzsch–Widman nomenclature, also called the extended Hantzsch–Widman system (named for Arthur Rudolf Hantzsch and ), is a type of systematic chemical nomenclature used for naming heterocyclic parent hydrides having no m ...
*International Union of Biochemistry and Molecular Biology
The International Union of Biochemistry and Molecular Biology (IUBMB) is an international non-governmental organisation concerned with biochemistry and molecular biology. Formed in 1955 as the International Union of Biochemistry (IUB), the union ...
*Nucleic acid notation
The nucleic acid notation currently in use was first formalized by the International Union of Pure and Applied Chemistry (IUPAC) in 1970. This universally accepted notation uses the Roman characters G, C, A, and T, to represent the four nucleotides ...
*Organic nomenclature in Chinese
The Chinese Chemical Society (CCS; ) lays out a set of rules based on those given by the International Union of Pure and Applied Chemistry (IUPAC) for the purpose of systematic organic nomenclature in Chinese. The chemical names derived from these ...
*Phanes
In Orphic cosmogony Phanes (, genitive ) or Protogonos () is a primeval deity who was born from the cosmic egg at the beginning of creation. He is referred by various names, including Erikepaios "Power" () and Metis "Thought".
Mythology ...
*Preferred IUPAC name
In chemical nomenclature, a preferred IUPAC name (PIN) is a unique name, assigned to a chemical substance and preferred among all possible names generated by IUPAC nomenclature. The "preferred IUPAC nomenclature" provides a set of rules for choo ...
*Von Baeyer nomenclature
In organic chemistry, the von Baeyer nomenclature is a system for describing Polycyclic compound, polycyclic (i.e. multi-Ring (chemistry), ringed) hydrocarbons. The system was originally developed in 1900 by German chemist Adolf von Baeyer for b ...
*IUPAC nomenclature of inorganic chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
References
Bibliography
*External links
IUPAC Nomenclature of Organic Chemistry
(online version of several older editions of the
IUPAC Blue Book
''Nomenclature of Organic Chemistry'', commonly referred to by chemists as the ''Blue Book'', is a collection of recommendations on organic chemical nomenclature published at irregular intervals by the International Union of Pure and Applied C ...
)IUPAC Recommendations on Organic & Biochemical Nomenclature, Symbols, Terminology, etc.
(includes IUBMB Recommendations for biochemistry)
(last updated 11 April 2003)
ACD/Name
Software for generating systematic nomenclature
ChemAxon Name <> Structure
– ChemAxon IUPAC (& traditional) name to structure and structure to IUPAC name software. As used a
chemicalize.org
A free web site/service that extracts IUPAC names from web pages and annotates a 'chemicalized' version with structure images. Structures from annotated pages can also be searched. *
* {{Organic chemistry Chemical nomenclature Encodings Organic chemistry