Brookhart's Acid on:
[Wikipedia]
[Google]
[Amazon]
Brookhart's acid is the salt of the
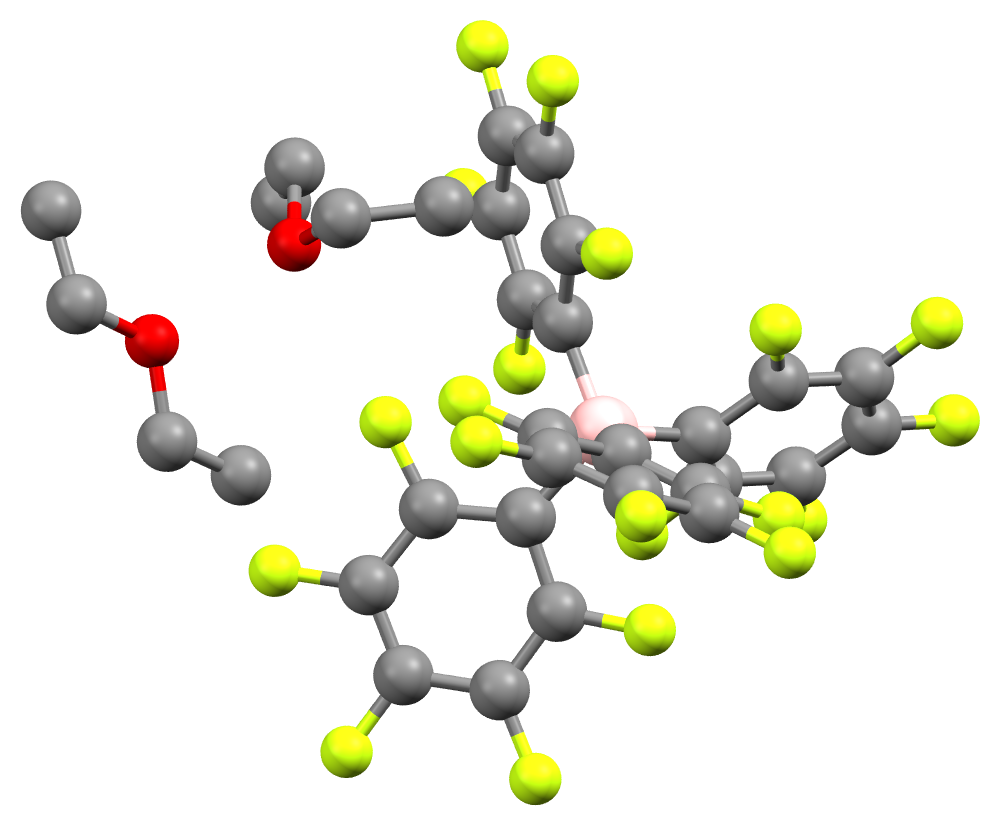 The acid crystallizes as a white, hygroscopic crystalline solid.
The acid crystallizes as a white, hygroscopic crystalline solid.

diethyl ether
Diethyl ether, or simply ether, is an organic compound with the chemical formula , sometimes abbreviated as . It is a colourless, highly Volatility (chemistry), volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It belongs ...
oxonium ion
In chemistry, an oxonium ion is any cation containing an oxygen atom that has three chemical bond, bonds and 1+ formal charge. The simplest oxonium ion is the hydronium ion ().
Alkyloxonium
Hydronium is one of a series of oxonium ions with the fo ...
and tetrakis ,5-bis(trifluoromethyl)phenylorate (BAr′4). It is a colorless solid, used as a strong acid. The compound was first reported by Volpe, Grant, and Brookhart in 1992.
Preparation
This compound is prepared by treatment of NaBAr′4 indiethyl ether
Diethyl ether, or simply ether, is an organic compound with the chemical formula , sometimes abbreviated as . It is a colourless, highly Volatility (chemistry), volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It belongs ...
(Et2O) with hydrogen chloride
The Chemical compound, compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hyd ...
:
: NaBAr′4 + HCl + 2 Et2O → (OEt2)2sup>+ + NaCl
NaBAr′4 is soluble in diethyl ether, whereas sodium chloride
Sodium chloride , commonly known as Salt#Edible salt, edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. It is transparent or translucent, brittle, hygroscopic, and occurs a ...
is not. Precipitation of sodium chloride thus drives the formation of the oxonium acid compound, which is isolable as a solid.
Structure and properties
 The acid crystallizes as a white, hygroscopic crystalline solid.
The acid crystallizes as a white, hygroscopic crystalline solid. NMR
Nuclear magnetic resonance (NMR) is a physical phenomenon in which atomic nucleus, nuclei in a strong constant magnetic field are disturbed by a weak oscillating magnetic field (in the near and far field, near field) and respond by producing ...
and elemental analysis
Elemental analysis is a process where a sample of some material (e.g., soil, waste or drinking water, bodily fluids, minerals, chemical compounds) is analyzed for its elemental and sometimes isotopic composition. Elemental analysis can be qualita ...
showed that the crystal contains two equivalents of diethyl ether. In solution, the compound slowly degrades to ''m''-C6H3(CF3)2 and BAr′3.
(OEt2)2B(C6F5)4] is a related compound with a slightly different weakly coordinating anion; it was first reported in 2000. An X-ray crystal structure of that compound was obtained, showing the acidic proton coordinated by both ethereal oxygen centers, although the crystal was not good enough to determine whether the proton is located symmetrically or unsymmetrically between the two.Jutzi, P.; Müller, C.; Stammler, A.; Stammler, H. G. (2000). "Synthesis, Crystal Structure, and Application of the Oxonium Acid (OEt2)2sup>+B(C6F5)4]−". Organometallics vol. 19, p. 1442.
Uses
Traditionalweakly coordinating anions
Anions that interact weakly with cations are termed non-coordinating anions, although a more accurate term is weakly coordinating anion. Non-coordinating anions are useful in studying the reactivity of electrophilic cations. They are commonly found ...
, such as perchlorate
A perchlorate is a chemical compound containing the perchlorate ion, , the conjugate base of perchloric acid (ionic perchlorate). As counterions, there can be metal cations, quaternary ammonium cations or other ions, for example, nitronium cat ...
, tetrafluoroborate
Tetrafluoroborate is the anion . This tetrahedral species is isoelectronic with tetrafluoroberyllate (), tetrafluoromethane (CF4), and tetrafluoroammonium () and is valence isoelectronic with many stable and important species including the perc ...
, and hexafluorophosphate
Hexafluorophosphate is an fluoroanion, anion with chemical formula of . It is an Octahedral molecular geometry, octahedral species that imparts no color to its salts. is isoelectronic with sulfur hexafluoride, , and the Hexafluorosilicic acid, h ...
, will nonetheless coordinate to very electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carr ...
cations, making these counterions unsuitable for some complexes. The highly reactive species p2Zr(CH3)sup>+, for example, has been reported to abstract F− from PF6. Starting in the 1980s, new types of weakly coordinating anions began to be developed. BAr′4 anions are used as counterions for highly electrophilic, cationic transition metal species, as they are very weakly coordinating and unreactive towards electrophilic attack. One common method of generating these cationic species is via protonolysis
Protonolysis is the cleavage of a chemical bond by acids. Many examples are found in organometallic chemistry since the reaction requires polar Mδ+-Rδ- bonds, where δ+ and δ- signify partial positive and negative charges associated with the bon ...
of a dialkyl complexes or an olefin
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as α-olefins.
The International Union of Pu ...
complex. For example, an electrophilic palladium
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), ...
catalyst, CH3CN)">acetonitrile.html" ;"title="2,2′-bipyridine)Pd(CH3)(acetonitrile">CH3CN)BAr′4], is prepared by protonating the dimethyl complex with Brookhart's acid. This electrophilic, cationic palladium species is used for the polymerization of olefins with carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
to polyketones in aprotic solvents.
Potential application
Polyketone
Polyketones are a family of high-performance thermoplastic polymers. The polar ketone groups in the polymer backbone of these materials gives rise to a strong attraction between polymer chains, which increases the material's melting point (255&nbs ...
s, thermoplastic
A thermoplastic, or thermosoftening plastic, is any plastic polymer material that becomes pliable or moldable at a certain elevated temperature and solidifies upon cooling.
Most thermoplastics have a high molecular weight. The polymer chains as ...
polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
s, are formed by the copolymerisation of carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
and one or more alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
s (typically ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon–carbon bond, carbon–carbon doub ...
with propylene
Propylene, also known as propene, is an unsaturated organic compound with the chemical formula . It has one double bond, and is the second simplest member of the alkene class of hydrocarbons. It is a colorless gas with a faint petroleum-like o ...
). The process utilises a palladium
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), ...
(II) catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
with a bidentate ligand like 2,2′-bipyridine
2,2′-Bipyridine (bipy or bpy, pronounced ) is an organic compound with the formula . This colorless solid is an important isomer of the bipyridine family. It is a bidentate chelation, chelating ligand, forming complexes with many transition meta ...
or 1,10-phenanthroline
1,10-Phenanthroline (phen) is a heterocyclic organic compound. It is a white solid that is soluble in organic solvents. The 1,10 refers to the location of the nitrogen atoms that replace CH's in the hydrocarbon called phenanthrene.
Abbreviated " ...
(phen) with a non-coordinating BARF counterion, such as phen)Pd(CH3)(CO)ArF4. The preparation of the catalyst involves the reaction of a dimethyl palladium complex with Brookhart's acid in acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not class ...
with loss of methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
and the catalytic species is formed by uptake of carbon monoxide to displace acetonitrile.
: Et2O)2HArF4 + phen)Pd(CH3)2 + MeCN → phen)Pd(CH3)(MeCN)ArF4 + 2 Et2O + CH4
: phen)Pd(CH3)(MeCN)ArF4 + CO → phen)Pd(CH3)(CO)ArF4 + MeCN
The mechanism involves migratory insertion
In organometallic chemistry, a migratory insertion is a type of chemical reaction, reaction wherein two ligands on a metal complex combine. It is a subset of reactions that very closely resembles the insertion reactions, and both are differentiate ...
whereby the polymer chain is bound to the catalytic centre and grows by the sequential insertion of carbon monoxide and the alkene between the palladium atom and the existing chain. Defects occur when insertions do not alternate – that is, a carbon monoxide insertion follows a carbon monoxide insertion or an alkene insertion follows an alkene insertion – these are highlighted in red in the figure below. This catalyst produces a very low rate of defects due to the difference in Gibbs energy of activation of each insertion – the energy barrier
In the Arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur. The activation energy (''E''a) of a reaction is measured in kilojoules per mole (k ...
to inserting an alkene immediately following an alkene insertion is ~12 kJ mol−1 higher than barrier to carbon monoxide insertion. Use of monodentate phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
ligands also leads to undesirable side-products but bidentate phosphine ligands like 1,3-bis(diphenylphosphino)propane
1,3-Bis(diphenylphosphino)propane (dppp) is an organophosphorus compound with the formula PhP(CH)PPh. The compound is a white solid that is soluble in organic solvents. It is slightly air-sensitive, degrading in air to the phosphine oxide. It is ...
have been used industrially.
References
{{Reflist Acids Non-coordinating anions Trifluoromethyl compounds Oxonium compounds