Borospherene on:
[Wikipedia]
[Google]
[Amazon]
 Borospherene (B40) is an electron-deficient cluster molecule containing 40
Borospherene (B40) is an electron-deficient cluster molecule containing 40
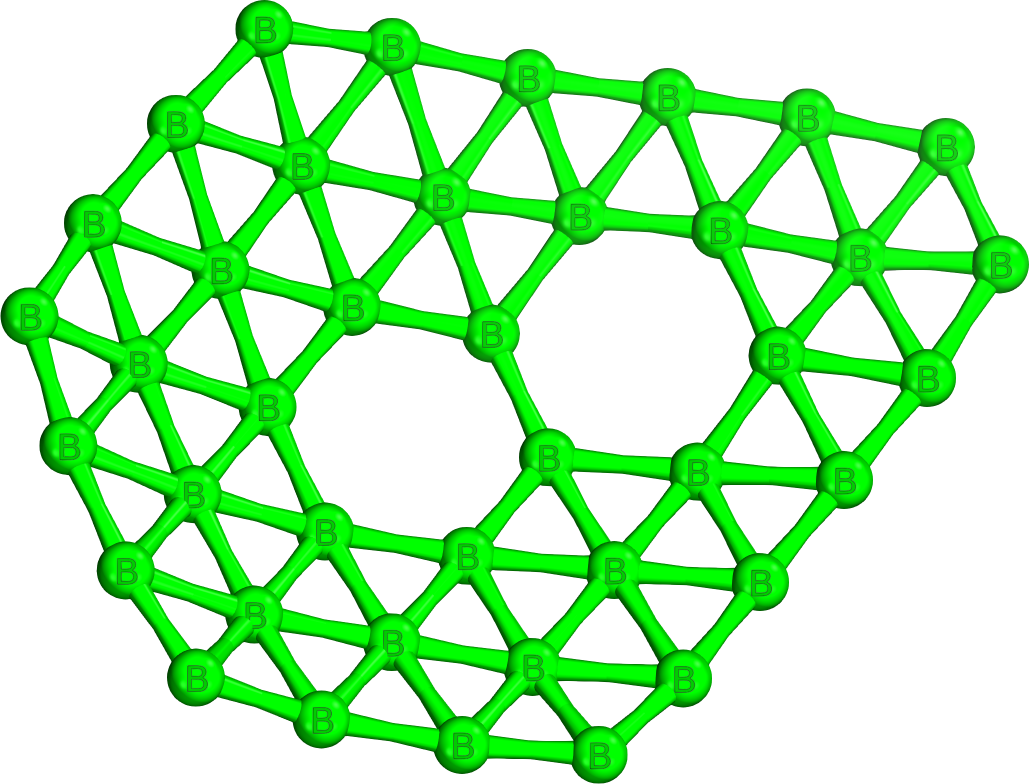
 In 2014, the first experimental evidence of a homoelemental
In 2014, the first experimental evidence of a homoelemental
 Borospherene (B40) is an electron-deficient cluster molecule containing 40
Borospherene (B40) is an electron-deficient cluster molecule containing 40 boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three ...
atoms. It bears similarities to other homoatomic cluster structures such as buckminsterfullerene
Buckminsterfullerene is a type of fullerene with the formula . It has a cage-like fused-ring structure ( truncated icosahedron) made of twenty hexagons and twelve pentagons, and resembles a football. Each of its 60 carbon atoms is bonded to i ...
(C60), stannaspherene, and plumbaspherene, but with a different symmetry. The first experimental evidence for borospherene was reported in July 2014, and is described in the journal ''Nature Chemistry
''Nature Chemistry'' is a monthly peer-reviewed scientific journal published by Nature Portfolio. It was established in April 2009. The editor-in-chief is Stuart Cantrill. The journal covers all aspects of chemistry. Publishing formats include prim ...
''. The molecule includes unusual hexagon
In geometry, a hexagon (from Greek , , meaning "six", and , , meaning "corner, angle") is a six-sided polygon. The total of the internal angles of any simple (non-self-intersecting) hexagon is 720°.
Regular hexagon
A regular hexagon is de ...
al and heptagon
In geometry, a heptagon or septagon is a seven-sided polygon or 7-gon.
The heptagon is sometimes referred to as the septagon, using ''Wikt:septa-, septa-'' (an elision of ''Wikt:septua-, septua-''), a Latin-derived numerical prefix, rather than ...
al faces. Despite many calculation-based investigations into its structure and properties, a viable route for the synthesis and isolation of borospherene has yet to be established, and as a consequence it is still relatively poorly understood.
History

 In 2014, the first experimental evidence of a homoelemental
In 2014, the first experimental evidence of a homoelemental fullerene
A fullerene is an allotropes of carbon, allotrope of carbon whose molecules consist of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to six atoms. The molecules may ...
-like B40 cluster was reported by Zhai ''et al''., after decades of theoretical investigations into boron cage structures following the discovery of buckminsterfullerene. Anionic B40− clusters were transiently produced by laser vaporisation of a 10B-enriched boron disc target, and studied with photoelectron spectroscopy. Their experimental spectrum corresponded well to a combination of simulated spectra of a sheet-like, quasi-planar global minimum
In mathematical analysis, the maximum and minimum of a function are, respectively, the greatest and least value taken by the function. Known generically as extremum, they may be defined either within a given range (the ''local'' or ''relative' ...
of the B40− anion (Cs symmetry) and its nearly degenerate fullerene-like structural isomer (D2d symmetry). However, the signal in the anion photoelectron spectrum assigned to cage-like B40 represents only a very minor fraction of the overall signal. Formation of cage-like B40, termed borospherene, has not been confirmed independently using any other experimental approach.
Many theoretical papers have been published on the structure, properties, and potential applications of borospherene. Neutral borospherene has a large HOMO-LUMO gap of 3.13 eV (which destabilises its anion, making the ground state of B40− the quasi-planar isomer). However, it has been calculated to be prone to exothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity (e ...
dimerisation, with a low activation barrier of 63 meV, followed by trimerisation with a lower energy barrier, and runaway aggregation. As a result, borospherene has yet to be isolated and is poorly experimentally-characterised, unlike buckminsterfullerene.
Structure
Borospherene has a unique C2 axis of symmetry, and belongs to the symmetry group is D2d (antiprismatic symmetry
In geometry, dihedral symmetry in three dimensions is one of three infinite sequences of point groups in three dimensions which have a symmetry group that as an abstract group is a dihedral group Dih''n'' (for ''n'' ≥ 2).
Types
Ther ...
, like a baseball
Baseball is a bat-and-ball games, bat-and-ball sport played between two team sport, teams of nine players each, taking turns batting (baseball), batting and Fielding (baseball), fielding. The game occurs over the course of several Pitch ...
) - in contrast to buckminsterfullerene, which has icosahedral symmetry
In mathematics, and especially in geometry, an object has icosahedral symmetry if it has the same symmetries as a regular icosahedron. Examples of other polyhedra with icosahedral symmetry include the regular dodecahedron (the dual polyhedr ...
. It features eight close-packed B6 triangles, two staggered hexagonal holes at its top and bottom, as well as four heptagonal holes along its sides. Unusually, the heptagons induce positive Gaussian curvature
In differential geometry, the Gaussian curvature or Gauss curvature of a smooth Surface (topology), surface in three-dimensional space at a point is the product of the principal curvatures, and , at the given point:
K = \kappa_1 \kappa_2.
For ...
(as opposed to negative Gaussian curvature in carbon nanotubes), which may play a role in strain reduction contributing to the stability of the cluster.
16 boron atoms of borospherene are four-coordinate, and 24 are five-coordinate. It has four sets of eight equivalent boron atoms, and two sets of four equivalent atoms.
Neutral borospherene has a diameter of 6.2 Å. It comprises eleven unique bond lengths ranging from 1.60 Å to 1.85 Å, corresponding to a B-B bond order of slightly below 2 to a fractional B-B bond order respectively. This encapsulates well the large degree of both sigma- and pi-delocalisation of electrons across the electron-deficient cluster as opposed to buckminsterfullerene, which has more localised bonds and features only two bond lengths corresponding to a C-C single bond and a C-C double bond respectively. The HOMO of borospherene is quadruply degenerate, computed to be a pi-bond delocalised over 5 boron atoms.
Lai-Sheng Wang, professor of chemistry at Brown University
Brown University is a Private university, private Ivy League research university in Providence, Rhode Island, United States. It is the List of colonial colleges, seventh-oldest institution of higher education in the US, founded in 1764 as the ' ...
, modeled possible B40 and B40− anion structures. The simulated spectra of two energetically lowest-lying isomers of the anion - a sheet-like structure and a closed cage - were found to fit experimental data well. Photoelectron spectroscopy revealed that the substance formed in the laboratory was this cage. Both neutral borospherene and the cage-like isomer of its anion have the same D2d symmetry, the additional electron in the anion being housed within the B40− cage structure. The structure of the cage is not perfectly uniform – "Several atoms stick out a bit from the others, making the surface of borospherene somewhat less smooth than a buckyball" according to Wang.
Potential applications
The cavity within the cage-like structure of borospherene, as well as borospherene's coordinatively unsaturated hexagonal and heptagonal faces, allows for the possibility of its endohedral or exohedral doping. With metal dopants, significant charge transfer is calculated to occur from the metals to the B40 cage - resulting in a positive charge forming on the metal, ostensibly making it capable of polarising small molecules. Such complexes formed are theorised to have applications in catalysis, and the detection or storage of small molecules such as H2.Small molecule sensing
Exploiting the thermal stability of B40 (calculated to be stable up to 1000 K), Liu ''et al''. investigated, with Van der Waals-correcteddensity functional theory
Density functional theory (DFT) is a computational quantum mechanical modelling method used in physics, chemistry and materials science to investigate the electronic structure (or nuclear structure) (principally the ground state) of many-body ...
calculations, the feasibility of using alkali metal-decorated B40 for the reversible storage and optical detection of hydrogen. Optimisation of (AM)6B40 structures (AM = Li, Na, K) revealed the metal atoms to be distributed above the centres of each hexagon and heptagon of B40, with a large binding energy in each case suggesting these complexes should be stable. H2 adsorption to these complexes induced a red-shift in their simulated TDDFT optical spectra in the case of Li6B40, and a blue-shift in the cases of Na6B40 and K6B40.
Li ''et al''. computationally investigated undecorated borospherene as a potential sensor for sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
-containing gases, and found that it behaved as an electronic sensor for sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a colorless gas with a pungent smell that is responsible for the odor of burnt matches. It is r ...
and carbon disulfide
Carbon disulfide (also spelled as carbon disulphide) is an inorganic compound with the chemical formula and structure . It is also considered as the anhydride of thiocarbonic acid. It is a colorless, flammable, neurotoxic liquid that is used as ...
(their adsorption to the boron cluster significantly stabilises its LUMO, increasing its population of conducting electrons), and additionally as a Φ-type sensor for the former (due to significant change to its work function Φ upon the adsorption of SO2), but behaved as neither for the gases carbonyl sulfide
Carbonyl sulfide is the chemical compound with the linear formula . It is a colorless flammable gas with an unpleasant odor. It is a linear molecule consisting of a carbonyl double bonded to a sulfur atom. Carbonyl sulfide can be considered to ...
and hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist ...
.
Small molecule storage
Modelling an exohedral Ca6B40, Esrafili ''et al''. simulatedcarbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
adsorption to the complex and found the upper bound of adsorption to be four CO2 molecules per Ca, with an average binding energy of -0.54 eV each - falling within the optimal range of binding energies for a CO2 adsorbent (0.40 - 0.80 eV), allowing facile desorption at elevated temperatures.
Undecorated B40 was calculated to be a poor candidate for reversible hydrogen storage, being capable of the irreversible sequestration of only one hydrogen molecule per B40 within its cage. Li6B40, however, is calculated to be capable of adsorbing up to 18 H2 molecules (3 H2 molecules at each Li site) - corresponding to a gravimetric density of 7.1 wt% - with a moderate average binding energy of 0.11 eV/H2, within the optimal range for reversible hydrogen storage. Subsequent H2 molecules are physisorbed to the cluster instead of chemisorbed, and have a much weaker binding energy.
See also
*Allotropes of boron
Boron can be prepared in several crystalline and amorphous forms. Well known crystalline forms are α-rhombohedral (α-R), β-rhombohedral (β-R), and β-tetragonal (β-T). In special circumstances, boron can also be synthesized in the form of ...
*Borophene
Borophene is a crystalline atomic monolayer of boron, i.e., it is a two-dimensional allotrope of boron and also known as ''boron sheet''.
First predicted by theory in the mid-1990s,
different borophene structures were experimentally confirmed ...
*Buckminsterfullerene
Buckminsterfullerene is a type of fullerene with the formula . It has a cage-like fused-ring structure ( truncated icosahedron) made of twenty hexagons and twelve pentagons, and resembles a football. Each of its 60 carbon atoms is bonded to i ...
References
External links
*{{Commons category-inline Allotropes of boron Fullerenes