Boron Hydride Cluster on:
[Wikipedia]
[Google]
[Amazon]
Boron hydride clusters are compounds with the formula or related anions, where x ≥ 3. Many such
Image:Borane-3D-balls.png, Borane
Image:Diborane-3D-balls-A.png, Diborane(6)
Image:Tetraborane-3D-balls.png, ''arachno''-Tetraborane(10)
Image:Pentaborane(9)-from-xtal-view-1-Mercury-3D-bs.png, Pentaborane(9)
Image:Decaborane(14)-from-xtal-view-1-tilt-3D-bs-17.png, Decaborane(14)
Image:B18H22 from Xray coordinates.tif, Octadecaborane(22)
Image:Iso-B18H22 from Xray coordinates.tif, ''iso''-
File:Hexaborate(6)-dianion-from-xtal-3D-bs-17.png, Hexaborate(6)
File:Heptaborate(7)-dianion-from-xtal-3D-bs-17.png, Heptaborate(7)
File:Octaborate(8)-dianion-from-xtal-3D-bs-17.png, Octaborate(8)
File:Nonaborate(9)-dianion-from-xtal-3D-bs-17.png, Nonaborate(9)
File:Decaborate(10)-dianion-from-xtal-3D-bs-17.png, Decaborate(10)
File:Closo-undecaborate(11)-dianion-from-xtal-3D-bs-17.png, Undecaborate(11)
Image:Dodecaborate(12)-dianion-from-xtal-3D-bs-17.png, Dodecaborate(12)
The naming of anions is illustrated by
:octahydridopentaborate,
The hydrogen count is specified first followed by the boron count. The -ate suffix is applied with
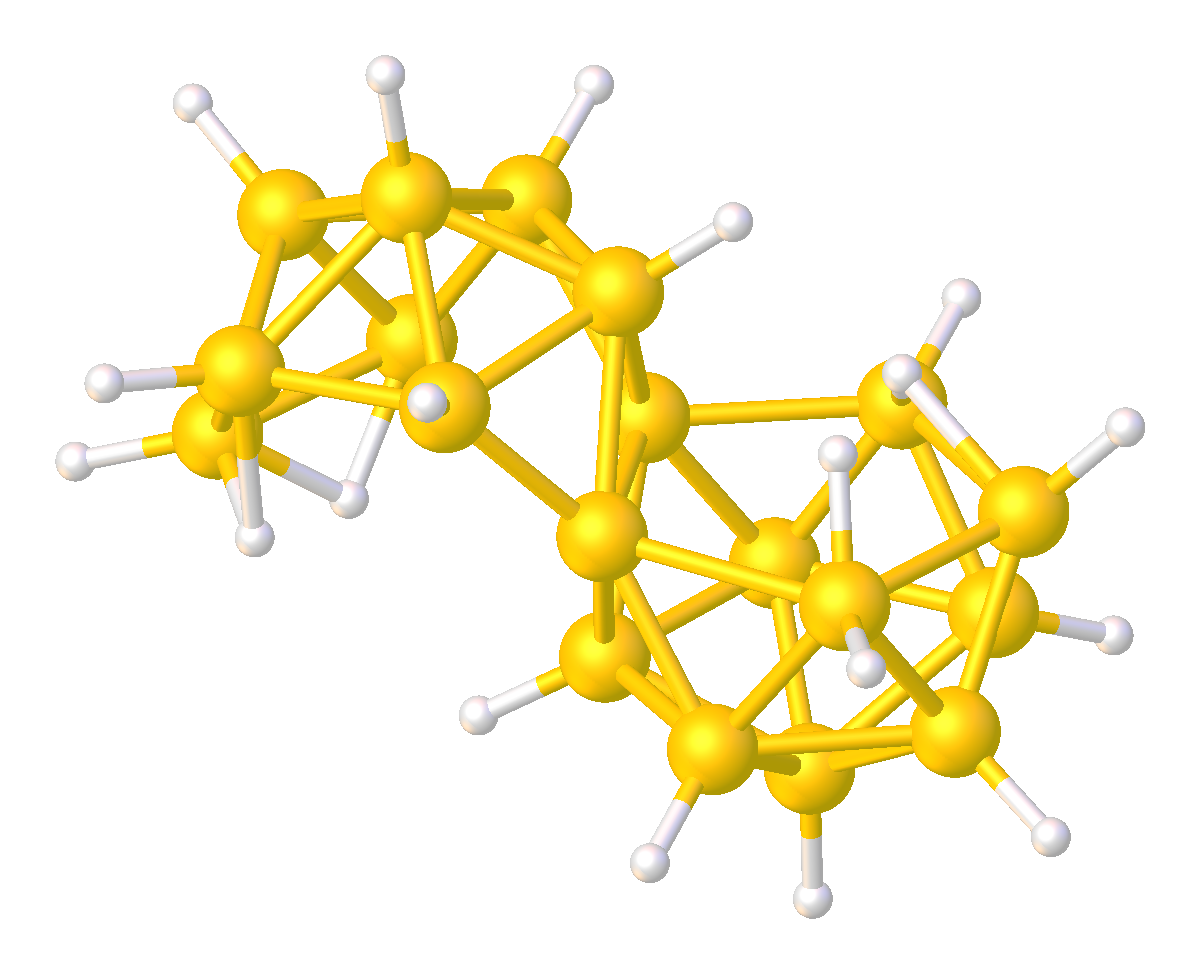 Although relatively rare, several multi-cluster boranes have been characterized. For example, reaction of a borane cluster with (as a source of ) can lead to the formation of a ''conjuncto''-borane species in which borane cluster sub-units are joined by the sharing of boron atoms.
:
:
Other ''conjuncto''-boranes, where the sub-units are joined by a B-B bond, can be made by ultra violet irradiation of ''nido''-boranes. Some B-B coupled ''conjuncto''-boranes can be produced using as catalyst.
Analogous to Wade's Rules, electron counting scheme has been developed to predict or rationalize multicluster boranes.
Although relatively rare, several multi-cluster boranes have been characterized. For example, reaction of a borane cluster with (as a source of ) can lead to the formation of a ''conjuncto''-borane species in which borane cluster sub-units are joined by the sharing of boron atoms.
:
:
Other ''conjuncto''-boranes, where the sub-units are joined by a B-B bond, can be made by ultra violet irradiation of ''nido''-boranes. Some B-B coupled ''conjuncto''-boranes can be produced using as catalyst.
Analogous to Wade's Rules, electron counting scheme has been developed to predict or rationalize multicluster boranes.
 For the boron hydride chemist, one of the most important reactions is the building up process by which smaller boron hydride clusters add borane to give larger clusters. This approach also applies to the synthesis of metallaboranes,
For the boron hydride chemist, one of the most important reactions is the building up process by which smaller boron hydride clusters add borane to give larger clusters. This approach also applies to the synthesis of metallaboranes,
cluster compound
Nanoclusters are atomically precise, crystalline materials most often existing on the 0-2 nanometer scale. They are often considered kinetically stable intermediates that form during the synthesis of comparatively larger materials such as semic ...
s are known. Common examples are those with 5, 10, and 12 boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three ...
atoms. Although they have few practical applications, the borane hydride clusters exhibit structures and bonding that differs strongly from the patterns seen in hydrocarbons. Hybrids of boranes and hydrocarbons, the carborane
Carboranes (or carbaboranes) are electron-delocalized (non-classically bonded) clusters composed of boron, carbon and hydrogen atoms.Grimes, R. N., ''Carboranes 3rd Ed.'', Elsevier, Amsterdam and New York (2016), . Like many of the related boron ...
s are also well developed.
History
The development of the borane hydride clusters resulted from pioneering work by Alfred Stock, invented the glass vacuum line for their study. The structures of the boron hydride clusters were determined beginning in 1948 with the characterization ofdecaborane
Decaborane, also called decaborane(14), is the inorganic compound with the chemical formula B10 H14. It is classified as a borane and more specifically a boron hydride cluster. This white crystalline compound is one of the principal boron hydri ...
. William Lipscomb was awarded the Nobel Prize in Chemistry
The Nobel Prize in Chemistry () is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outst ...
in 1976 for this and many subsequent crystallographic investigations. These investigations revealed the prevalence of deltahedral structures, i.e., networks of triangular arrays of BH centers.
The bonding of the clusters ushered in Polyhedral skeletal electron pair theory and Wade's rules, which can be used to predict the structures of boranes. These rules were found to describe structures of many cluster compounds.
Chemical formula and naming conventions
Borane clusters are classified as follows, where ''n'' is the number of boron atoms in a single cluster: pp 151-195 The International Union of Pure and Applied Chemistry rules for systematic naming is based on a prefix denoting a class of compound, followed by the number of boron atoms and finally the number of hydrogen atoms in parentheses. Various details can be omitted if there is no ambiguity about the meaning, for example, if only one structural type is possible. Some examples of the structures are shown below.Image:Diborane-3D-balls-A.png, Diborane(6)
Image:Tetraborane-3D-balls.png, ''arachno''-Tetraborane(10)
Image:Pentaborane(9)-from-xtal-view-1-Mercury-3D-bs.png, Pentaborane(9)
Image:Decaborane(14)-from-xtal-view-1-tilt-3D-bs-17.png, Decaborane(14)
Image:B18H22 from Xray coordinates.tif, Octadecaborane(22)
Image:Iso-B18H22 from Xray coordinates.tif, ''iso''-
File:Heptaborate(7)-dianion-from-xtal-3D-bs-17.png, Heptaborate(7)
File:Octaborate(8)-dianion-from-xtal-3D-bs-17.png, Octaborate(8)
File:Nonaborate(9)-dianion-from-xtal-3D-bs-17.png, Nonaborate(9)
File:Decaborate(10)-dianion-from-xtal-3D-bs-17.png, Decaborate(10)
File:Closo-undecaborate(11)-dianion-from-xtal-3D-bs-17.png, Undecaborate(11)
Image:Dodecaborate(12)-dianion-from-xtal-3D-bs-17.png, Dodecaborate(12)
anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
s. The ionic charge value is included in the chemical formula but not as part of the systematic name.
Bonding in boranes
Boranes are nonclassically–bonded compounds, that is, there are not enough electrons to form 2-centre, 2-electron bonds between all pairs of adjacent atoms in the molecule. A description of the bonding in the larger boranes was formulated by William Lipscomb. It involved: * 3-center 2-electron B-H-B hydrogen bridges *3-center 2-electron B-B-B bonds *2-center 2-electron bonds (in B-B, B-H and ) Lipscomb's methodology has largely been superseded by a molecular orbital approach. This allows the concept of multi-centre bonding to be extended. For example, in the icosahedral ion , the totally symmetric (Ag symmetry) molecular orbital is equally distributed among all 12 boron atoms. Wade's rules provide a powerful method that can be used to rationalize the structures in terms of the number of atoms and the connectivity between them.Multicluster boranes
 Although relatively rare, several multi-cluster boranes have been characterized. For example, reaction of a borane cluster with (as a source of ) can lead to the formation of a ''conjuncto''-borane species in which borane cluster sub-units are joined by the sharing of boron atoms.
:
:
Other ''conjuncto''-boranes, where the sub-units are joined by a B-B bond, can be made by ultra violet irradiation of ''nido''-boranes. Some B-B coupled ''conjuncto''-boranes can be produced using as catalyst.
Analogous to Wade's Rules, electron counting scheme has been developed to predict or rationalize multicluster boranes.
Although relatively rare, several multi-cluster boranes have been characterized. For example, reaction of a borane cluster with (as a source of ) can lead to the formation of a ''conjuncto''-borane species in which borane cluster sub-units are joined by the sharing of boron atoms.
:
:
Other ''conjuncto''-boranes, where the sub-units are joined by a B-B bond, can be made by ultra violet irradiation of ''nido''-boranes. Some B-B coupled ''conjuncto''-boranes can be produced using as catalyst.
Analogous to Wade's Rules, electron counting scheme has been developed to predict or rationalize multicluster boranes.
Lewis acid/base behavior
Some function as electron donors owing to the relative basic character of the groups. Boranes can function asligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
s in coordination compound
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ' ...
s. Hapticities of η1 to η6 have been found, with electron donation involving bridging H atoms or donation from B-B bonds. For example, ''nido-'' can replace ethene in Zeise's salt to produce .
They can also act as Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any ...
s, with concomitant opening of the cluster. An example involving trimethylphosphine:
:
Brønsted acid/base behavior
Some higher boranes, especially those with bridging hydrogen atoms, can be deprotonated with a strong base. An example: : Acidity increases with the size of the borane, with having a p''K''a value of 2.7: : In general, bridging hydrogen protons tend to be lost before terminal ones.Aufbau reactions
 For the boron hydride chemist, one of the most important reactions is the building up process by which smaller boron hydride clusters add borane to give larger clusters. This approach also applies to the synthesis of metallaboranes,
For the boron hydride chemist, one of the most important reactions is the building up process by which smaller boron hydride clusters add borane to give larger clusters. This approach also applies to the synthesis of metallaboranes,
Hydroboration
Reminiscent of the behavior of diborane and its adducts, higher boranes participate in hydroboration. When boron hydrides add analkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
, the carbon becomes incorporated into the cluster, producing carborane
Carboranes (or carbaboranes) are electron-delocalized (non-classically bonded) clusters composed of boron, carbon and hydrogen atoms.Grimes, R. N., ''Carboranes 3rd Ed.'', Elsevier, Amsterdam and New York (2016), . Like many of the related boron ...
s, e.g. .
Applications
Some cobalt derivatives of carboranes have been commercialized for sequestering fromradioactive waste
Radioactive waste is a type of hazardous waste that contains radioactive material. It is a result of many activities, including nuclear medicine, nuclear research, nuclear power generation, nuclear decommissioning, rare-earth mining, and nuclear ...
.
Boranes have a high specific energy
Specific energy or massic energy is energy per unit mass. It is also sometimes called gravimetric energy density, which is not to be confused with energy density, which is defined as energy per unit volume. It is used to quantify, for example, st ...
of combustion compared to hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually fain ...
s, making them potentially attractive as fuels or igniters. Intense research was carried out in the 1950s into their use as jet fuel additives, but the effort did not lead to practical results.
Aspirational uses
Because has a very high neutron-capture cross section, boron-hydride derivatives have often been investigated for applications in Neutron capture therapy of cancer. : (2.4 Mev)See also
* :Boranes, containing all specific borane-compound articlesReferences
{{Authority control