Boron-19 on:
[Wikipedia]
[Google]
[Amazon]
[] , p , Subsequently decays by double proton emission to for a net reaction of → + 3 , (3/2−) , , , - , Has 1 halo nucleus, halo protonIntermediate product of Proton–proton chain#The p–p III branch, a branch of proton–proton chain in stellar nucleosynthesis as part of the process converting hydrogen to helium , style="text-align:center" , 5 , style="text-align:center" , 3 , , , β+ α , , 2+ , , , -id=Boron-8m , style="text-indent:1em" , , colspan="3" style="text-indent:2em" , , , , , 0+ , , , -id=Boron-9 , , style="text-align:center" , 5 , style="text-align:center" , 4 , , , p , , Immediately decays into two α particles, for a net reaction of → 2 + , 3/2− , , , - , One of the few stable odd-odd nuclei , style="text-align:center" , 5 , style="text-align:center" , 5 , , colspan=3 align=center, Stable , 3+ , colspan=2 align=center, ref name="Atomic Weight of Boron2"> , -id=Boron-11 , , style="text-align:center" , 5 , style="text-align:center" , 6 , , colspan=3 align=center, Stable , 3/2− , colspan=2 align=center, ref name="Atomic Weight of Boron2" /> , -id=Boron-11m , style="text-indent:1em" , , colspan="3" style="text-indent:2em" , , , , , 1/2+, (3/2+) , , , -id=Boron-12 , rowspan=2, , rowspan=2 style="text-align:center" , 5 , rowspan=2 style="text-align:center" , 7 , rowspan=2, , rowspan=2, , β− () , , rowspan=2, 1+ , rowspan=2, , rowspan=2, , - , β−α () , Immediately decays into two α particles, for a net reaction of → 3 + , -id=Boron-13 , rowspan=2, , rowspan=2 style="text-align:center" , 5 , rowspan=2 style="text-align:center" , 8 , rowspan=2, , rowspan=2, , β− () , , rowspan=2, 3/2− , rowspan=2, , rowspan=2, , - , β−n () , , -id=Boron-14 , rowspan=3, , rowspan=3 style="text-align:center" , 5 , rowspan=3 style="text-align:center" , 9 , rowspan=3, , rowspan=3, , β− () , , rowspan=3, 2− , rowspan=3, , rowspan=3, , - , β−n () , , - , β−2n ?Decay mode shown is energetically allowed, but has not been experimentally observed to occur in this nuclide. , ? , -id=Boron-14m , style="text-indent:1em" , , colspan="3" style="text-indent:2em" , , , IT ? , , 0+ , , , -id=Boron-15 , rowspan=3, , rowspan=3 style="text-align:center" , 5 , rowspan=3 style="text-align:center" , 10 , rowspan=3, , rowspan=3, , β−n () , , rowspan=3, 3/2− , rowspan=3, , rowspan=3, , - , β− (< ) , , - , β−2n (< ) , , -id=Boron-16 , , style=text-align:center , 5 , style=text-align:center , 11 , , >
Boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three ...
(5B) naturally occurs as isotopes
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), but ...
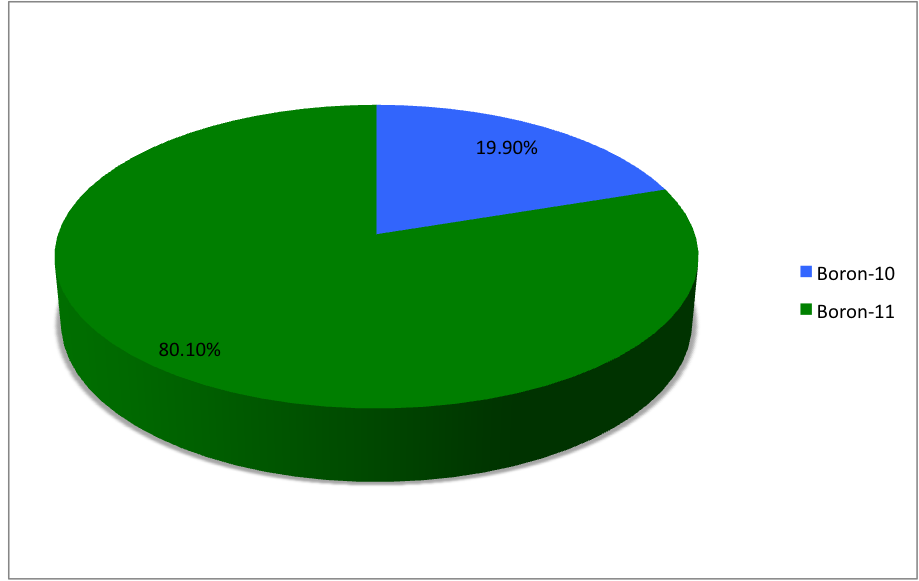
and , the latter of which makes up about 80% of natural boron. There are 13 radioisotopes
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess numbers of either neutrons or protons, giving it excess nuclear energy, and making it unstable. This excess energy can be used in one of three ...
that have been discovered, with mass numbers from 7 to 21, all with short half-lives Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* '' Half Life: A Parable for t ...
, the longest being that of , with a half-life of only and with a half-life of . All other isotopes have half-lives shorter than . Those isotopes with mass below 10 decay into helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is ...
(via short-lived isotopes of beryllium
Beryllium (4Be) has 11 known Isotope, isotopes and 3 known nuclear isomer, isomers, but only one of these isotopes () is stable and a primordial nuclide. As such, beryllium is considered a monoisotopic element. It is also a mononuclidic elemen ...
for and ) while those with mass above 11 mostly become carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
.

List of isotopes
, -id=Boron-7 , , style="text-align:center" , 5 , style="text-align:center" , 2 , ,[] , p , Subsequently decays by double proton emission to for a net reaction of → + 3 , (3/2−) , , , - , Has 1 halo nucleus, halo protonIntermediate product of Proton–proton chain#The p–p III branch, a branch of proton–proton chain in stellar nucleosynthesis as part of the process converting hydrogen to helium , style="text-align:center" , 5 , style="text-align:center" , 3 , , , β+ α , , 2+ , , , -id=Boron-8m , style="text-indent:1em" , , colspan="3" style="text-indent:2em" , , , , , 0+ , , , -id=Boron-9 , , style="text-align:center" , 5 , style="text-align:center" , 4 , , , p , , Immediately decays into two α particles, for a net reaction of → 2 + , 3/2− , , , - , One of the few stable odd-odd nuclei , style="text-align:center" , 5 , style="text-align:center" , 5 , , colspan=3 align=center, Stable , 3+ , colspan=2 align=center, ref name="Atomic Weight of Boron2"> , -id=Boron-11 , , style="text-align:center" , 5 , style="text-align:center" , 6 , , colspan=3 align=center, Stable , 3/2− , colspan=2 align=center, ref name="Atomic Weight of Boron2" /> , -id=Boron-11m , style="text-indent:1em" , , colspan="3" style="text-indent:2em" , , , , , 1/2+, (3/2+) , , , -id=Boron-12 , rowspan=2, , rowspan=2 style="text-align:center" , 5 , rowspan=2 style="text-align:center" , 7 , rowspan=2, , rowspan=2, , β− () , , rowspan=2, 1+ , rowspan=2, , rowspan=2, , - , β−α () , Immediately decays into two α particles, for a net reaction of → 3 + , -id=Boron-13 , rowspan=2, , rowspan=2 style="text-align:center" , 5 , rowspan=2 style="text-align:center" , 8 , rowspan=2, , rowspan=2, , β− () , , rowspan=2, 3/2− , rowspan=2, , rowspan=2, , - , β−n () , , -id=Boron-14 , rowspan=3, , rowspan=3 style="text-align:center" , 5 , rowspan=3 style="text-align:center" , 9 , rowspan=3, , rowspan=3, , β− () , , rowspan=3, 2− , rowspan=3, , rowspan=3, , - , β−n () , , - , β−2n ?Decay mode shown is energetically allowed, but has not been experimentally observed to occur in this nuclide. , ? , -id=Boron-14m , style="text-indent:1em" , , colspan="3" style="text-indent:2em" , , , IT ? , , 0+ , , , -id=Boron-15 , rowspan=3, , rowspan=3 style="text-align:center" , 5 , rowspan=3 style="text-align:center" , 10 , rowspan=3, , rowspan=3, , β−n () , , rowspan=3, 3/2− , rowspan=3, , rowspan=3, , - , β− (< ) , , - , β−2n (< ) , , -id=Boron-16 , , style=text-align:center , 5 , style=text-align:center , 11 , , >