Biosimilar Insulins on:
[Wikipedia]
[Google]
[Amazon]
 An insulin analogue ( also called an insulin analog) is a type of medical insulin that has been modified to alter its pharmacokinetic properties while maintaining the same biological function as
An insulin analogue ( also called an insulin analog) is a type of medical insulin that has been modified to alter its pharmacokinetic properties while maintaining the same biological function as
 Short-acting insulin analogues are modified forms of recombinant human insulin designed to enhance subcutaneous absorption and accelerate glycemic control. In standard insulin formulations, regular insulin monomers naturally aggregate into hexamers, a configuration that delays absorption and prolongs the onset of action. Before entering the bloodstream, these hexamers must dissociate into dimers and then monomers, which slows their availability for glucose regulation. To address this limitation, insulin analogues have been engineered to maintain a monomeric or dimeric configuration, allowing for faster absorption and reducing the time to onset to approximately 5 to 15 minutes. Insulin lispro, insulin aspart, and insulin glulisine are the most widely used short-acting insulin analogues. These formulations are structurally identical to human insulin, except for amino acid substitutions at one or two positions, which modify their stability and absorption characteristics.
Insulin lispro, which was first approved in 1996 and marketed as Humalog among others, works by reversing the final
Short-acting insulin analogues are modified forms of recombinant human insulin designed to enhance subcutaneous absorption and accelerate glycemic control. In standard insulin formulations, regular insulin monomers naturally aggregate into hexamers, a configuration that delays absorption and prolongs the onset of action. Before entering the bloodstream, these hexamers must dissociate into dimers and then monomers, which slows their availability for glucose regulation. To address this limitation, insulin analogues have been engineered to maintain a monomeric or dimeric configuration, allowing for faster absorption and reducing the time to onset to approximately 5 to 15 minutes. Insulin lispro, insulin aspart, and insulin glulisine are the most widely used short-acting insulin analogues. These formulations are structurally identical to human insulin, except for amino acid substitutions at one or two positions, which modify their stability and absorption characteristics.
Insulin lispro, which was first approved in 1996 and marketed as Humalog among others, works by reversing the final 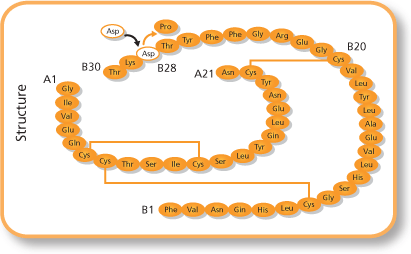 insulin can reduce hypoglycemia incidence and improve glycemic control. Insulin aspart, which was approved in 2000 and is marketed under the name Novolog among others, has effects comparable to those of insulin lispro, but has a lesser risk of nocturnal hypoglycemia. It works by replacing a proline with an
insulin can reduce hypoglycemia incidence and improve glycemic control. Insulin aspart, which was approved in 2000 and is marketed under the name Novolog among others, has effects comparable to those of insulin lispro, but has a lesser risk of nocturnal hypoglycemia. It works by replacing a proline with an
 Insulin degludec, marketed as Tresiba and approved in 2015, is an ultra-long-acting insulin with a duration of up to 42 hours. It utilizes multi-hexamer formation and albumin binding to provide a steady insulin release with lower intra-individual variability and greater dosing flexibility. Compared to insulin glargine and detemir, degludec offers a reduced risk of nocturnal hypoglycemia and allows dosing intervals of 8 to 40 hours without compromising glycemic control. These advancements have improved diabetes management by providing more stable blood sugar control, fewer hypoglycemic episodes, and greater convenience for patients.
Insulin icodec is, as of 2025, the newest and longest-acting insulin analogue. It has a plasma half-life that is more than eight days, meaning it is a once-weekly insulin. It was approved in 2024 and is marketed as Awuqli by Novo Nordisk. Insulin icodec consists of two peptide chains linked by a
Insulin degludec, marketed as Tresiba and approved in 2015, is an ultra-long-acting insulin with a duration of up to 42 hours. It utilizes multi-hexamer formation and albumin binding to provide a steady insulin release with lower intra-individual variability and greater dosing flexibility. Compared to insulin glargine and detemir, degludec offers a reduced risk of nocturnal hypoglycemia and allows dosing intervals of 8 to 40 hours without compromising glycemic control. These advancements have improved diabetes management by providing more stable blood sugar control, fewer hypoglycemic episodes, and greater convenience for patients.
Insulin icodec is, as of 2025, the newest and longest-acting insulin analogue. It has a plasma half-life that is more than eight days, meaning it is a once-weekly insulin. It was approved in 2024 and is marketed as Awuqli by Novo Nordisk. Insulin icodec consists of two peptide chains linked by a
 The development of insulin therapy has progressed significantly since the early 20th century, starting with animal-derived insulins. In 1922, Frederick Banting and Charles Best successfully used bovine insulin extract to treat humans for the first time. This breakthrough led to the commercial production of bovine insulin in 1923 by Eli Lilly and Company. That same year, Hans Christian Hagedorn founded the Nordisk Insulinlaboratorium in Denmark, which later became Novo Nordisk. In 1926, Nordisk received a Danish charter to produce insulin as a non-profit entity. In 1936, Canadian researchers D.M. Scott and A.M. Fisher developed a zinc insulin mixture, which was licensed to Novo. During this time, Hagedorn discovered that adding protamine to insulin could prolong its action, which led to the development of Neutral Protamine Hagedorn (NPH) insulin in 1946. NPH insulin was marketed by Nordisk in 1950. By 1953, Novo also developed Lente insulin by adding zinc to porcine and bovine insulins, resulting in a longer-acting form.
A significant advancement in insulin production occurred in 1978 when Genentech developed the biosynthesis of recombinant human insulin using Escherichia coli bacteria and recombinant DNA technology. This allowed for the production of insulin identical to that produced by the human pancreas. In 1981, Novo Nordisk chemically and enzymatically converted porcine insulin into human insulin. Genentech's synthetic human insulin, produced in partnership with Eli Lilly, was approved by the U.S. Food and Drug Administration in 1982. Lilly's biosynthetic recombinant insulin, branded as Humulin, was introduced in 1983. In 1985, Axel Ullrich sequenced the human insulin receptor, further enhancing the understanding of insulin's biological mechanisms. By 1988, Novo Nordisk produced synthetic recombinant human insulin, which further improved insulin availability and consistency.
The development of insulin therapy has progressed significantly since the early 20th century, starting with animal-derived insulins. In 1922, Frederick Banting and Charles Best successfully used bovine insulin extract to treat humans for the first time. This breakthrough led to the commercial production of bovine insulin in 1923 by Eli Lilly and Company. That same year, Hans Christian Hagedorn founded the Nordisk Insulinlaboratorium in Denmark, which later became Novo Nordisk. In 1926, Nordisk received a Danish charter to produce insulin as a non-profit entity. In 1936, Canadian researchers D.M. Scott and A.M. Fisher developed a zinc insulin mixture, which was licensed to Novo. During this time, Hagedorn discovered that adding protamine to insulin could prolong its action, which led to the development of Neutral Protamine Hagedorn (NPH) insulin in 1946. NPH insulin was marketed by Nordisk in 1950. By 1953, Novo also developed Lente insulin by adding zinc to porcine and bovine insulins, resulting in a longer-acting form.
A significant advancement in insulin production occurred in 1978 when Genentech developed the biosynthesis of recombinant human insulin using Escherichia coli bacteria and recombinant DNA technology. This allowed for the production of insulin identical to that produced by the human pancreas. In 1981, Novo Nordisk chemically and enzymatically converted porcine insulin into human insulin. Genentech's synthetic human insulin, produced in partnership with Eli Lilly, was approved by the U.S. Food and Drug Administration in 1982. Lilly's biosynthetic recombinant insulin, branded as Humulin, was introduced in 1983. In 1985, Axel Ullrich sequenced the human insulin receptor, further enhancing the understanding of insulin's biological mechanisms. By 1988, Novo Nordisk produced synthetic recombinant human insulin, which further improved insulin availability and consistency.
ImageSize = width:800 height:auto barincrement:22
PlotArea = left:170 bottom:65 top:30 right:20
Alignbars = justify
DateFormat = mm/dd/yyyy
Period = from:01/01/1995 till:12/01/2025
TimeAxis = orientation:horizontal format:yyyy
Colors =
id:short value:rgb(1,0.0,0) legend:Short-acting
id:long value:rgb(0,0.4,1) legend:Long-acting
id:shortb value:rgb(1,0.6,0.8) legend:Short-acting_biosimilar
id:longb value:rgb(0,0.8,1) legend:Long-acting_biosimilar
id:bars value:gray(1)
id:grid1 value:rgb(0.5,0.5,0.5)
id:grid2 value:gray(0.92)
Legend = orientation:vertical position:bottom
BackgroundColors = bars:bars
ScaleMajor = increment:5 start:1995 gridcolor:grid1
ScaleMinor = increment:1 start:1995 gridcolor:grid2
TextData=
pos:(300,272) fontsize:M text:Insulin analogue and biosimilar approvals, 1995-2025
BarData =
bar:Lispro text:Insulin_lispro
bar:Glargine text:Insulin_glargine
bar:Aspart text:Insulin_aspart
bar:Glulisine text:Insulin_glulisine
bar:Detemir text:Insulin_detemir
bar:Degludec text:Insulin_degludec
bar:Glargine-yfgn text:Insulin_glargine-yfgn
bar:Glargine-aglr text:Insulin_glargine-aglr
bar:Icodec text:Insulin_icodec
bar:Aspart-szjj text:Insulin_aspart-szjj
PlotData=
width:10 textcolor:black align:left anchor:from shift:(10,-4)
bar:Lispro width:15 from:06/14/1996 till:12/01/2025 color:short
bar:Glargine width:15 from:04/20/2000 till:12/01/2025 color:long
bar:Aspart width:15 from:06/07/2000 till:12/01/2025 color:short
bar:Glulisine width:15 from:04/16/2004 till:12/01/2025 color:short
bar:Detemir width:15 from:06/16/2005 till:12/01/2025 color:long
bar:Degludec width:15 from:09/25/2015 till:12/01/2025 color:long
bar:Glargine-yfgn width:15 from:07/28/2021 till:12/01/2025 color:longb
bar:Glargine-aglr width:15 from:12/01/2021 till:12/01/2025 color:longb
bar:Icodec width:15 from:03/20/2024 till:12/01/2025 color:long
bar:Aspart-szjj width:15 from:02/14/2025 till:12/01/2025 color:shortb
human insulin
As a medication, insulin is any pharmaceutical preparation of the protein hormone insulin that is used to treat high blood glucose. Such conditions include type 1 diabetes, type 2 diabetes, gestational diabetes, and complications of diabetes ...
. These modifications are achieved through genetic engineering
Genetic engineering, also called genetic modification or genetic manipulation, is the modification and manipulation of an organism's genes using technology. It is a set of Genetic engineering techniques, technologies used to change the genet ...
, which allows for changes in the amino acid sequence
Protein primary structure is the linear sequence of amino acids in a peptide or protein. By convention, the primary structure of a protein is reported starting from the amino-terminal (N) end to the carboxyl-terminal (C) end. Protein biosynthe ...
of insulin to optimize its absorption, distribution, metabolism, and excretion (ADME
ADME is the four-letter abbreviation (acronym) for absorption (pharmacokinetics), ''absorption'', distribution (pharmacology), ''distribution'', ''metabolism'', and ''excretion'', and is mainly used in fields such as pharmacokinetics and pharmacol ...
) characteristics.
All insulin analogues work by enhancing glucose uptake in tissues and reducing glucose production by the liver
The liver is a major metabolic organ (anatomy), organ exclusively found in vertebrates, which performs many essential biological Function (biology), functions such as detoxification of the organism, and the Protein biosynthesis, synthesis of var ...
. They are prescribed for conditions such as type 1 diabetes
Type 1 diabetes (T1D), formerly known as juvenile diabetes, is an autoimmune disease that occurs when the body's immune system destroys pancreatic cells (beta cells). In healthy persons, beta cells produce insulin. Insulin is a hormone require ...
, type 2 diabetes
Type 2 diabetes (T2D), formerly known as adult-onset diabetes, is a form of diabetes mellitus that is characterized by high blood sugar, insulin resistance, and relative lack of insulin. Common symptoms include increased thirst, frequent ...
, gestational diabetes
Gestational diabetes is a condition in which a woman without diabetes develops hyperglycemia, high blood sugar levels during pregnancy. Gestational diabetes generally results in few symptoms. Obesity increases the rate of pre-eclampsia, cesarea ...
, and diabetes-related complications such as diabetic ketoacidosis
Diabetic ketoacidosis (DKA) is a potentially life-threatening acute complication of diabetes mellitus. Signs and symptoms may include vomiting, abdominal pain, deep gasping breathing, increased urination, weakness, confusion and occasionally ...
. Additionally, insulin is sometimes administered alongside glucose to treat elevated blood potassium levels (hyperkalemia
Hyperkalemia is an elevated level of potassium (K+) in the blood. Normal potassium levels are between 3.5 and 5.0 mmol/L (3.5 and 5.0 mEq/L) with levels above 5.5mmol/L defined as hyperkalemia. Typically hyperkalemia does not cause symptoms. Oc ...
).
Insulin analogues are classified based on their duration of action
Pharmacodynamics (PD) is the study of the biochemical and physiologic effects of drugs (especially pharmaceutical drugs). The effects can include those manifested within animals (including humans), microorganisms, or combinations of organisms (fo ...
. Short-acting (bolus) insulin analogues, such as insulin lispro
Insulin lispro, sold under the brand name Humalog among others, is a modified type of medical insulin used to treat type 1 and type 2 diabetes. It is delivered subcutaneously either by injection or from an insulin pump. Onset of effects t ...
, insulin aspart, and insulin glulisine
Insulin glulisine, sold under the brand name Apidra among others, is a rapid-acting modified form of medical insulin used for the treatment of diabetes. It differs from human insulin in that the amino acid asparagine at position B3 is replace ...
, have been designed to be absorbed quickly, mimicking the natural insulin response after meals. Long-acting (basal) insulin analogues, including insulin glargine
Insulin glargine sold under the brand name Lantus among others is a long-acting modified form of medical insulin, used in the management of type 1 and type 2 diabetes. It is injected just under the skin. Effects generally begin an hour af ...
, insulin detemir, and insulin degludec, provide a sustained release of insulin to maintain basal blood glucose levels over an extended period. These modifications enhance the predictability of insulin therapy and reduce the risk of hypoglycemia
Hypoglycemia (American English), also spelled hypoglycaemia or hypoglycæmia (British English), sometimes called low blood sugar, is a fall in blood sugar to levels below normal, typically below 70 mg/dL (3.9 mmol/L). Whipple's tria ...
compared to regular human insulin.
Lispro, the first insulin analogue, was approved in 1996. This was followed by an influx of new analogues with differing pharmacokinetic properties. The first long-acting analogue, insulin glargine, was approved in 2000. Insulin aspart, insulin glulisine, and insulin detemir were all approved by 2005. The second wave of insulin analogues, which include insulin degludec and insulin icodec, started in the mid-2010s.
Mechanisms of action
Insulin analogues arerecombinant proteins
Recombinant DNA (rDNA) molecules are DNA molecules formed by laboratory methods of genetic recombination (such as molecular cloning) that bring together genetic material from multiple sources, creating sequences that would not otherwise be found ...
that are structurally based on human insulin but have been modified through amino acid substitutions or additions to alter their pharmacokinetic properties. These modifications are designed to either accelerate or prolong subcutaneous absorption while maintaining the biological function of insulin in regulating blood glucose levels. Native human insulin, commonly referred to as regular insulin
Regular insulin, also known as neutral insulin and soluble insulin, is a type of short-acting medical insulin. It is used to treat type 1 diabetes, type 2 diabetes, gestational diabetes, and complications of diabetes such as diabetic ketoaci ...
, naturally assembles into hexamers
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relative ...
, which must gradually dissociate into dimers and then monomer
A monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called polymerization.
Classification
Chemis ...
s before they can be absorbed into the bloodstream. This process results in a delayed onset of action
Onset of action is the duration of time it takes for a drug's effects to come to prominence upon administration. With oral administration, it typically ranges anywhere from 20 minutes to over an hour, depending on the drug in question. Other meth ...
, making the timing of insulin administration a critical factor in diabetes management
Diabetes mellitus is a metabolic disease that is characterized by chronic elevated blood glucose levels (hyperglycemia). Therefore, the main goal of diabetes management is to keep blood glucose levels within normal limits or a target range as ...
.
short-acting insulin analogues are developed to have a shorter duration of action than regular insulin, while long-acting insulin analogues are meant to have a peakless action profile and a prolonged duration of action.
Short-acting
 Short-acting insulin analogues are modified forms of recombinant human insulin designed to enhance subcutaneous absorption and accelerate glycemic control. In standard insulin formulations, regular insulin monomers naturally aggregate into hexamers, a configuration that delays absorption and prolongs the onset of action. Before entering the bloodstream, these hexamers must dissociate into dimers and then monomers, which slows their availability for glucose regulation. To address this limitation, insulin analogues have been engineered to maintain a monomeric or dimeric configuration, allowing for faster absorption and reducing the time to onset to approximately 5 to 15 minutes. Insulin lispro, insulin aspart, and insulin glulisine are the most widely used short-acting insulin analogues. These formulations are structurally identical to human insulin, except for amino acid substitutions at one or two positions, which modify their stability and absorption characteristics.
Insulin lispro, which was first approved in 1996 and marketed as Humalog among others, works by reversing the final
Short-acting insulin analogues are modified forms of recombinant human insulin designed to enhance subcutaneous absorption and accelerate glycemic control. In standard insulin formulations, regular insulin monomers naturally aggregate into hexamers, a configuration that delays absorption and prolongs the onset of action. Before entering the bloodstream, these hexamers must dissociate into dimers and then monomers, which slows their availability for glucose regulation. To address this limitation, insulin analogues have been engineered to maintain a monomeric or dimeric configuration, allowing for faster absorption and reducing the time to onset to approximately 5 to 15 minutes. Insulin lispro, insulin aspart, and insulin glulisine are the most widely used short-acting insulin analogues. These formulations are structurally identical to human insulin, except for amino acid substitutions at one or two positions, which modify their stability and absorption characteristics.
Insulin lispro, which was first approved in 1996 and marketed as Humalog among others, works by reversing the final lysine
Lysine (symbol Lys or K) is an α-amino acid that is a precursor to many proteins. Lysine contains an α-amino group (which is in the protonated form when the lysine is dissolved in water at physiological pH), an α-carboxylic acid group ( ...
and proline
Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group but is rather a secondary amine. The secondary amine nitrogen is in the p ...
residues on the C-terminal end of the B-chain. This modification does not alter receptor binding, but blocks the formation of insulin dimers and hexamers. Clinical studies have demonstrated that the use of insulin lispro instead of regular
 insulin can reduce hypoglycemia incidence and improve glycemic control. Insulin aspart, which was approved in 2000 and is marketed under the name Novolog among others, has effects comparable to those of insulin lispro, but has a lesser risk of nocturnal hypoglycemia. It works by replacing a proline with an
insulin can reduce hypoglycemia incidence and improve glycemic control. Insulin aspart, which was approved in 2000 and is marketed under the name Novolog among others, has effects comparable to those of insulin lispro, but has a lesser risk of nocturnal hypoglycemia. It works by replacing a proline with an aspartic acid
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. The L-isomer of aspartic acid is one of the 22 proteinogenic amino acids, i.e., the building blocks of protei ...
at the B28 position. Insulin glulisine has nearly identical properties to the other two short-acting analogues, but differs in the fact that the amino acid asparagine
Asparagine (symbol Asn or N) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), an α-carboxylic acid group (which is in the depro ...
at position B3 is replaced by lysine and the lysine in position B29 is replaced by glutamic acid
Glutamic acid (symbol Glu or E; known as glutamate in its anionic form) is an α- amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can ...
. It was approved in 2004 and is sold under the name Apidra.
These short-acting insulin analogues play a crucial role in modern diabetes management, as their fast onset and shorter duration of action allow for more precise postprandial glucose control. By closely mimicking endogenous
Endogeny, in biology, refers to the property of originating or developing from within an organism, tissue, or cell.
For example, ''endogenous substances'', and ''endogenous processes'' are those that originate within a living system (e.g. an ...
insulin secretion, these analogues enhance glycemic stability, reduce post-meal blood sugar spikes, and minimize the risk of hypoglycemic events. Their pharmacokinetic properties make them particularly beneficial for individuals requiring flexible meal timing and those using intensive insulin therapy.
Long-acting
Long-acting insulin analogues are designed to provide continuous basal insulin coverage for up to 24 hours, with the exception of ultra-long-acting analogues, which work for up to a week. These include insulin glargine, insulin detemir, insulin degludec, and insulin icodec, which have been modified through amino acid substitutions andfatty acid
In chemistry, in particular in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated and unsaturated compounds#Organic chemistry, saturated or unsaturated. Most naturally occurring fatty acids have an ...
conjugation to alter their subcutaneous absorption and extend their duration of action. A key feature of long-acting insulin analogues is reversible albumin
Albumin is a family of globular proteins, the most common of which are the serum albumins. All of the proteins of the albumin family are water- soluble, moderately soluble in concentrated salt solutions, and experience heat denaturation. Alb ...
binding and di-hexamer formation, which slow insulin dissociation and provide a more stable pharmacokinetic and pharmacodynamic
Pharmacodynamics (PD) is the study of the biochemistry, biochemical and physiology, physiologic effects of drugs (especially pharmaceutical drugs). The effects can include those manifested within animals (including humans), microorganisms, or comb ...
profile, reducing glycemic fluctuations and nocturnal hypoglycemia.
Insulin glargine (100 U/mL), first approved by the FDA
The United States Food and Drug Administration (FDA or US FDA) is a federal agency of the Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food ...
in 2000 and marketed as Lantus, forms zinc-mediated hexamer aggregates after injection, resulting in a slow insulin release. In 2015, a higher-concentration formulation (300 U/mL), marketed as Toujeo, was introduced, offering up to 36-hour coverage and a lower risk of nocturnal hypoglycemia. Insulin detemir, approved in 2005 as Levemir, features a C14 fatty acid modification at lysine B29, promoting di-hexamer formation and albumin binding for an extended duration. While effective, insulin detemir often requires twice-daily dosing for optimal glycemic control.
 Insulin degludec, marketed as Tresiba and approved in 2015, is an ultra-long-acting insulin with a duration of up to 42 hours. It utilizes multi-hexamer formation and albumin binding to provide a steady insulin release with lower intra-individual variability and greater dosing flexibility. Compared to insulin glargine and detemir, degludec offers a reduced risk of nocturnal hypoglycemia and allows dosing intervals of 8 to 40 hours without compromising glycemic control. These advancements have improved diabetes management by providing more stable blood sugar control, fewer hypoglycemic episodes, and greater convenience for patients.
Insulin icodec is, as of 2025, the newest and longest-acting insulin analogue. It has a plasma half-life that is more than eight days, meaning it is a once-weekly insulin. It was approved in 2024 and is marketed as Awuqli by Novo Nordisk. Insulin icodec consists of two peptide chains linked by a
Insulin degludec, marketed as Tresiba and approved in 2015, is an ultra-long-acting insulin with a duration of up to 42 hours. It utilizes multi-hexamer formation and albumin binding to provide a steady insulin release with lower intra-individual variability and greater dosing flexibility. Compared to insulin glargine and detemir, degludec offers a reduced risk of nocturnal hypoglycemia and allows dosing intervals of 8 to 40 hours without compromising glycemic control. These advancements have improved diabetes management by providing more stable blood sugar control, fewer hypoglycemic episodes, and greater convenience for patients.
Insulin icodec is, as of 2025, the newest and longest-acting insulin analogue. It has a plasma half-life that is more than eight days, meaning it is a once-weekly insulin. It was approved in 2024 and is marketed as Awuqli by Novo Nordisk. Insulin icodec consists of two peptide chains linked by a disulfide
In chemistry, a disulfide (or disulphide in British English) is a compound containing a functional group or the anion. The linkage is also called an SS-bond or sometimes a disulfide bridge and usually derived from two thiol groups.
In inorg ...
bridge. It contains a C20 fatty diacid
In organic chemistry, a dicarboxylic acid is an organic compound containing two carboxyl groups (). The general molecular formula for dicarboxylic acids can be written as , where R can be aliphatic or aromatic.Boy Cornils, Peter Lappe "Dicarboxyli ...
-containing side chain, which facilitates strong, reversible binding to albumin. Additionally, three amino acid substitutions are introduced to enhance molecular stability, reduce insulin receptor binding, and slow clearance. These modifications collectively contribute to the prolonged half-life.
Side effects
The most common side effect in all insulin analogues is low blood sugar, while in more serious cases, side effects may include low blood potassium. Insulin allergies are also a concern, although they are not prevalent, affecting only about 2% of people in some form. Insulin analogues are generally considered safe duringpregnancy
Pregnancy is the time during which one or more offspring gestation, gestates inside a woman's uterus. A multiple birth, multiple pregnancy involves more than one offspring, such as with twins.
Conception (biology), Conception usually occurs ...
, and many are used in the treatment of gestational diabetes
Gestational diabetes is a condition in which a woman without diabetes develops hyperglycemia, high blood sugar levels during pregnancy. Gestational diabetes generally results in few symptoms. Obesity increases the rate of pre-eclampsia, cesarea ...
.
Carcinogenicity
All insulin analogs undergocarcinogen
A carcinogen () is any agent that promotes the development of cancer. Carcinogens can include synthetic chemicals, naturally occurring substances, physical agents such as ionizing and non-ionizing radiation, and biologic agents such as viruse ...
icity testing due to insulin's interaction with IGF (insulin-like growth factor
The insulin-like growth factors (IGFs) are proteins with high sequence similarity to insulin. IGFs are part of a complex system that cells use to communicate with their physiologic environment. This complex system (often referred to as the IGF ...
) pathways, which can promote abnormal cell growth and tumorigenesis
Carcinogenesis, also called oncogenesis or tumorigenesis, is the formation of a cancer, whereby normal cells are transformed into cancer cells. The process is characterized by changes at the cellular, genetic, and epigenetic levels and abn ...
. Structural modifications to insulin always carry the risk of unintentionally enhancing IGF signaling, potentially increasing mitogen
A mitogen is a small bioactive protein or peptide that induces a cell to begin cell division, or enhances the rate of division (mitosis). Mitogenesis is the induction (triggering) of mitosis, typically via a mitogen.
The cell cycle
Mitogens a ...
ic activity alongside the intended pharmacological effects. Concerns have been raised specifically regarding the carcinogenic potential of insulin glargine, prompting several epidemiological
Epidemiology is the study and analysis of the distribution (who, when, and where), patterns and Risk factor (epidemiology), determinants of health and disease conditions in a defined population, and application of this knowledge to prevent dise ...
studies to investigate its safety.
Comparison with other insulins
NPH
Neutral Protamine Hagedorn (NPH) insulin, or isophane insulin, is an intermediate-acting insulin developed in 1946 to extend insulin activity through the addition ofprotamine
Protamines are small, arginine-rich, nuclear proteins that replace histones late in the haploid phase of spermatogenesis and are believed essential for sperm head condensation and DNA stabilization. They may allow for denser packaging of DNA ...
, which slows absorption. It has an onset of about 90 minutes and lasts up to 24 hours, making it suitable for once- or twice-daily administration. NPH insulin is available as a recombinant human insulin and is sometimes premixed with short-acting insulin for combined basal and mealtime glucose control.
During the 1980s, many individuals experienced difficulties when transitioning to intermediate-acting insulins, particularly NPH formulations of porcine
The pig (''Sus domesticus''), also called swine (: swine) or hog, is an omnivorous, domesticated, even-toed, hoofed mammal. It is named the domestic pig when distinguishing it from other members of the genus '' Sus''. Some authorities consid ...
and bovine
Bovines (subfamily Bovinae) comprise a diverse group of 10 genera of medium to large-sized ungulates, including Bos, cattle, bison, African buffalo, Bubalus, water buffalos, and the four-horned and spiral-horned antelopes. The members of this gro ...
insulins.Owens DR, Bolli GB. 2008 Beyond the era of NPH insulin--long-acting insulin analogs: chemistry, comparative pharmacology, and clinical application. Diabetes Technol Ther. Oct;10(5):333-49. These issues stemmed from variability in absorption and inconsistent glucose control. In response, basal insulin analogues were developed to provide a more stable and predictable absorption profile,Jonassen I, Havelund S, Hoeg-Jensen T, Steensgaard DB, Wahlund PO, Ribel U. 2012 Design of the novel protraction mechanism of insulin degludec, an ultra-long-acting basal insulin. Pharm Res. 2012 Aug;29(8):2104-14. leading to improved clinical efficacy and glycemic management.
Animal-derived insulins
Animal insulins, including porcine and bovine insulin, were the first clinically used insulins, extracted from the pancreas of animals before the availability of biosynthetic human insulin (insulin human rDNA). Porcine insulin differs from human insulin by a single amino acid, while bovine insulin has three variations, yet both exhibit similar activity at the human insulin receptor. Prior to the introduction of biosynthetic insulin, shark-derived insulin was commonly used in Japan, and certain fish insulins were also found to be effective in humans. While non-human insulins were widely used, they sometimes triggered allergic reactions, primarily due to impurities and preservatives in insulin preparations. Although the formation of non-neutralizing antibodies was rare, some patients experienced immune responses that affected insulin efficacy. The development of biosynthetic human insulin significantly reduced these issues, leading to its widespread adoption and largely replacing animal-derived insulin in clinical practice.Biosimilar insulin
Abiosimilar
A biosimilar (also known as follow-on biologic or subsequent entry biologic) is a biologic medical product that is almost an identical copy of an original product that is manufactured by a different company. Biosimilars are officially approved ...
is a biological medicine that is highly similar to an already approved reference biologic in terms of structure, biological activity, efficacy, and safety. These medicines are large, complex molecules produced through biotechnology
Biotechnology is a multidisciplinary field that involves the integration of natural sciences and Engineering Science, engineering sciences in order to achieve the application of organisms and parts thereof for products and services. Specialists ...
in living systems, such as microorganisms, plant cells, or animal cells. Due to differences in the manufacturing process, biosimilars cannot be exact copies of reference biologics but must demonstrate high similarity through extensive structural and functional analysis. Manufacturers are required to show that a biosimilar has no clinically meaningful differences from its reference product regarding safety, purity, and potency, which is assessed through pharmacokinetic (PK) and pharmacodynamic (PD) studies, immunogenicity evaluations, and, if necessary, additional clinical studies. Biosimilars can only be developed and marketed once the patent on the original reference biologic has expired, allowing for competition and increased availability of biologic therapies.
The expiration of patents for first-generation insulin analogs has facilitated the development of biosimilar insulins, offering potential to improve global insulin access. Despite the essential role of insulin, approximately half of individuals who require it do not have access due to high costs and limited availability. This issue is particularly pronounced in low-income countries, where economic factors can restrict the use of biologic treatments such as insulin. Biosimilar insulins, which have a shorter development timeline of about eight years compared to 12 years for novel biologic drugs, provide a more affordable alternative, with development costs ranging from 10% to 20% of those for new biologics. These products could help improve access to treatment and reduce disparities in insulin availability.
The global market for biologic medicines, including insulin, grew from $46 billion in 2002 to $390 billion in 2020, accounting for 28% of the global pharmaceutical market. In the United States, biologics represented 43% of drug expenditures, totaling $211 billion in 2019, with biosimilar spending expected to rise from $5.2 billion in 2019 to nearly $27 billion by 2024. In Europe, biologics accounted for 34% of medicine spending, reaching US$78.6 billion in 2021, with the biosimilar market valued at $8.8 billion. The global human insulin market was valued at $22.9 billion in 2020, while the biosimilar insulin market stood at $2.3 billion, projected to grow to $5.6 billion by 2027. The introduction of biosimilar insulins has increased market competition, offering a cost-effective alternative that could lower treatment costs and reduce strain on healthcare systems.
Since the approval of the first biosimilar insulin, interest in the products has increased. However, uncertainty regarding their safety and efficacy has slowed their adoption among healthcare professionals. Regulatory agencies, such as the European Medicines Agency
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of pharmaceutical products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products ...
(EMA) and the U.S. Food and Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a federal agency of the Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food ...
(FDA), have established approval pathways to ensure biosimilar insulins meet the same quality, safety, and efficacy standards as reference products.
Available biosimilars
As of 2025, there are three commercially available biosimilar insulins. They are insulin glargine-yfgn, insulin glargine-aglr, and insulin aspart-szjj. Insulin glargine-yfgn is marketed under the name Semglee, and reveived FDA approval in July 2021, but development began before that. The approval was granted toMylan
Mylan N.V. was a global generic and specialty pharmaceuticals company. In November 2020, Mylan merged with Upjohn, Pfizer's off-patent medicine division, to form Viatris. Previously, the company was domiciled in the Netherlands, with principa ...
, which was merged with another company into Viatris
Viatris Inc. is an American global pharmaceutical and healthcare corporation headquartered in Canonsburg, Pennsylvania. The corporation was formed through the merger of Mylan and Upjohn, a legacy division of Pfizer, on November 16, 2020.
The ...
in 2020. The second approved biosimilar insulin, insulin glargine-aglr, was approved by the FDA in December 2021 to be produced by Lilly under the name Rezvoglar. In February, 2025, the FDA approved the very first short-acting biosimilar insulin, insulin aspart szjj. It is manufactured by Viatris and sold under the name Merilog.
It is of note that although the name of insulin lispro-aabc, which is marketed as Lyumjev by Lilly, is similar to the names of biosimilars, it is not a biosimilar insulin. Insulin lispro-aabc is simply a faster formulation of insulin lispro.
Modifications
Before biosynthetic human recombinant analogues became available, porcine insulin was chemically modified to create human insulin. These semisynthetic insulins were produced by altering amino acid side chains at theN-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amin ...
and C-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, carboxy tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein
Proteins are large biomolecules and macromolecules that comp ...
to modify absorption, distribution, metabolism, and excretion (ADME) characteristics. Novo Nordisk
Novo Nordisk A/S is a Danish multinational pharmaceutical company headquartered in Bagsværd, with production facilities in nine countries and affiliates or offices in five. Novo Nordisk is controlled by majority shareholder Novo Holdings A/S ...
developed one such method by enzymatically converting porcine insulin into human insulin by replacing the single differing amino acid. Unmodified human and porcine insulins naturally form hexamers with zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic tabl ...
, requiring dissociation into monomers before binding to insulin receptors. This delays insulin activity when injected subcutaneously, making it less effective for postprandial glucose control.
Basal insulin analogues were developed with altered isoelectric point
The isoelectric point (pI, pH(I), IEP), is the pH at which a molecule carries no net electric charge, electrical charge or is electrically neutral in the statistical mean. The standard nomenclature to represent the isoelectric point is pH(I). Howe ...
s, allowing them to precipitate at physiological pH and dissolve slowly, providing insulin coverage for up to 24 hours. Some, like insulin detemir, bind to albumin rather than fat, prolonging their action. Non-hexameric (monomeric) insulins were later introduced for faster-acting mealtime coverage, mimicking naturally occurring monomeric insulins found in certain animal species. These advancements in insulin formulation allowed for greater flexibility in diabetes management, with basal insulin analogues providing steady background insulin levels and short-acting analogues offering improved postprandial glucose control.
Zinc-complexed insulins continued to be used for slow-release basal support, covering approximately 50% of daily insulin needs, while mealtime insulin made up the remaining half. The development of monomeric insulins addressed the limitations of hexameric formulations, ensuring faster absorption and better glycemic control. As research progressed, insulin analogues with enhanced receptor binding, extended duration, and improved stability became standard in modern diabetes treatment, reducing variability in glucose levels and lowering the risk of hypoglycemia.
History
Early insulins (1922–1995)
 The development of insulin therapy has progressed significantly since the early 20th century, starting with animal-derived insulins. In 1922, Frederick Banting and Charles Best successfully used bovine insulin extract to treat humans for the first time. This breakthrough led to the commercial production of bovine insulin in 1923 by Eli Lilly and Company. That same year, Hans Christian Hagedorn founded the Nordisk Insulinlaboratorium in Denmark, which later became Novo Nordisk. In 1926, Nordisk received a Danish charter to produce insulin as a non-profit entity. In 1936, Canadian researchers D.M. Scott and A.M. Fisher developed a zinc insulin mixture, which was licensed to Novo. During this time, Hagedorn discovered that adding protamine to insulin could prolong its action, which led to the development of Neutral Protamine Hagedorn (NPH) insulin in 1946. NPH insulin was marketed by Nordisk in 1950. By 1953, Novo also developed Lente insulin by adding zinc to porcine and bovine insulins, resulting in a longer-acting form.
A significant advancement in insulin production occurred in 1978 when Genentech developed the biosynthesis of recombinant human insulin using Escherichia coli bacteria and recombinant DNA technology. This allowed for the production of insulin identical to that produced by the human pancreas. In 1981, Novo Nordisk chemically and enzymatically converted porcine insulin into human insulin. Genentech's synthetic human insulin, produced in partnership with Eli Lilly, was approved by the U.S. Food and Drug Administration in 1982. Lilly's biosynthetic recombinant insulin, branded as Humulin, was introduced in 1983. In 1985, Axel Ullrich sequenced the human insulin receptor, further enhancing the understanding of insulin's biological mechanisms. By 1988, Novo Nordisk produced synthetic recombinant human insulin, which further improved insulin availability and consistency.
The development of insulin therapy has progressed significantly since the early 20th century, starting with animal-derived insulins. In 1922, Frederick Banting and Charles Best successfully used bovine insulin extract to treat humans for the first time. This breakthrough led to the commercial production of bovine insulin in 1923 by Eli Lilly and Company. That same year, Hans Christian Hagedorn founded the Nordisk Insulinlaboratorium in Denmark, which later became Novo Nordisk. In 1926, Nordisk received a Danish charter to produce insulin as a non-profit entity. In 1936, Canadian researchers D.M. Scott and A.M. Fisher developed a zinc insulin mixture, which was licensed to Novo. During this time, Hagedorn discovered that adding protamine to insulin could prolong its action, which led to the development of Neutral Protamine Hagedorn (NPH) insulin in 1946. NPH insulin was marketed by Nordisk in 1950. By 1953, Novo also developed Lente insulin by adding zinc to porcine and bovine insulins, resulting in a longer-acting form.
A significant advancement in insulin production occurred in 1978 when Genentech developed the biosynthesis of recombinant human insulin using Escherichia coli bacteria and recombinant DNA technology. This allowed for the production of insulin identical to that produced by the human pancreas. In 1981, Novo Nordisk chemically and enzymatically converted porcine insulin into human insulin. Genentech's synthetic human insulin, produced in partnership with Eli Lilly, was approved by the U.S. Food and Drug Administration in 1982. Lilly's biosynthetic recombinant insulin, branded as Humulin, was introduced in 1983. In 1985, Axel Ullrich sequenced the human insulin receptor, further enhancing the understanding of insulin's biological mechanisms. By 1988, Novo Nordisk produced synthetic recombinant human insulin, which further improved insulin availability and consistency.
Initial analogue development (1996–2014)
The development of insulin analogues began with Humalog (insulin lispro), a short-acting insulin analogue developed byEli Lilly
Eli Lilly (July 8, 1838 – June 6, 1898) was a Union Army officer, pharmacist, chemist, and businessman who founded Eli Lilly and Company.
Lilly enlisted in the Union Army during the American Civil War and recruited a company of men to ...
, which was approved by the FDA in 1996. Humalog was designed to be absorbed more quickly than regular insulin, offering improved flexibility in meal timing and postprandial glucose control. In 2000, Lantus (insulin glargine) was approved by the FDA and the European Medicines Agency
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of pharmaceutical products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products ...
(EMA). Lantus is a long-acting insulin analogue designed to provide a steady basal level of insulin throughout the day, typically lasting up to 24 hours, thereby reducing the need for multiple daily injections. In 2004, Apidra (insulin glulisine), another short-acting insulin analog, was approved by Sanofi-Aventis
Sanofi S.A. is a French multinational pharmaceutical and healthcare company headquartered in Paris, France. The corporation was established in 1973 and merged with Synthélabo in 1999 to form Sanofi-Synthélabo. In 2004, Sanofi-Synthélabo merg ...
to improve postprandial glucose control.
In 2005, Levemir (insulin detemir), developed by Novo Nordisk, was approved for clinical use. Levemir is a long-acting insulin analogue similar to Lantus but with a slightly shorter duration of action. It provides stable basal insulin coverage with a reduced risk of hypoglycemia compared to older insulins.
Modern analogues (2015–present)
As of 2025, many companies are researching and manufacturing new insulin analogues. These insulins are usually designed to be either ultra-short-acting or ultra-long-acting. Insulin degludec, an ultra-long-acting insulin analog, was developed by Novo Nordisk and approved by the FDA in 2015. Insulin degludec has an extended duration of action, lasting up to 42 hours, offering greater flexibility in dosing schedules. In March 2024, insulin icodec was approved for medical use in Canada. The same month, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) issued a positive opinion, recommending the granting of marketing authorization for Awiqli, under which insulin icodec is marketed. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged. Following the CHMP's recommendation, insulin icodec was approved for medical use in the European Union in May 2024. Insulin icodec has a plasma half-life more than eight days (compared to 25 hours of the previous longest-acting insulin analogue insulin degludec), making it a once-weekly basal insulin.Experimental analogues
Insulin tregopil is an experimental ultra-fast-acting insulin that is being developed by Biocon. Unlike other insulin analogues, it is designed to be taken orally. It has been modified with the covalent attachment of amethoxy
In organic chemistry, a methoxy group is the functional group consisting of a methyl group bound to oxygen. This alkoxy group has the formula .
On a benzene ring, the Hammett equation classifies a methoxy substituent at the ''para'' position a ...
- triethylene-glycol- propionyl moiety
Moiety may refer to:
__NOTOC__ Anthropology
* Moiety (kinship), either of two groups into which a society is divided
** A division of society in the Iroquois societal structure in North America
** An Australian Aboriginal kinship group
** Native Ha ...
at Lys-β29-amino group of the B-chain. This modification, along with the use of sodium caprate as a permeation enhancer, allows insulin tregopil to be absorbed through the gastrointestinal tract
The gastrointestinal tract (GI tract, digestive tract, alimentary canal) is the tract or passageway of the Digestion, digestive system that leads from the mouth to the anus. The tract is the largest of the body's systems, after the cardiovascula ...
. Another oral analogue called ORMD-0801 is, as of 2025, in development by Oramed Pharmaceuticals.
Insulin efsitora alfa is an experimental insulin analogue developed by Eli Lilly for the treatment of diabetes. Its glycemic control and safety were found to be similar to insulin degludec in a phase II clinical trial.
NNC2215 is a bioengineered glucose-sensitive insulin analogue developed by Novo Nordisk researchers. The drug is designed to adjust its activity based on blood glucose levels, reducing insulin sensitivity when glucose concentrations are low, thereby lowering the risk of hypoglycemia. It also provides more stable blood sugar control by responding dynamically to fluctuations in glucose levels. A study on NNC2215 was published in the journal ''Nature
Nature is an inherent character or constitution, particularly of the Ecosphere (planetary), ecosphere or the universe as a whole. In this general sense nature refers to the Scientific law, laws, elements and phenomenon, phenomena of the physic ...
'' on October 16, 2024, describing its potential as a major advancement in diabetes treatment and the role of protein engineering in future medicine. The development of glucose-sensitive insulin has been an area of interest in diabetes research since 1979, aiming to address blood sugar fluctuations. Several previous attempts have been made to create glucose-responsive insulin, with varying degrees of success.
In the 2010s, Eli Lilly and Company developed an experimental basal insulin analogue called peglispro (BIL), which showed a prolonged and flat activity profile with hepato-preferential action. Although BIL demonstrated improved glycemic control, reduced nocturnal hypoglycemia, and less weight gain compared to insulin glargine, it was associated with increased liver fat, triglyceride
A triglyceride (from '' tri-'' and '' glyceride''; also TG, triacylglycerol, TAG, or triacylglyceride) is an ester derived from glycerol and three fatty acids.
Triglycerides are the main constituents of body fat in humans and other vertebrates ...
s, and liver enzyme levels. Due to these concerns and the uncertain regulatory pathway, Lilly discontinued the development of BIL in 2015.
Other experimental analogues that are in development include LAPS Insulin115, an ultralong analogue being researched by Hanmi Pharm, and two basal oral analogues in development by Novo Nordisk, OI338 and OI320.
Approval overview
Since 1996, seven novel insulin analogues have been approved. Three short-acting and four long-acting analogues have been made, while one short-acting lispro modification has been produced. Since 2021, three insulin biosimilars have been approved, two of which are long-acting and one of which is short-acting. * 1996: ''Insulin lispro'', which was originally manufactured by Eli Lilly and Company, is granted approval. * 2000: ''Insulin aspart'', which was created by Novo Nordisk, is approved. * 2000: ''Insulin glargine'', which was developed by Sanofi-Aventis, is approved. * 2004: ''Insulin glulisine'', also developed by Sanofi-Aventis, is approved. * 2005: ''Insulin detemir'', which was formulated by Novo Nordisk, gets approval. * 2015: ''Insulin degludec,'' created by Novo Nordisk, is approved. * 2020: ''Insulin lispro-aabc'', a faster insulin lispro formulation created by Elly Lilly and Company, is approved. * 2021: ''Insulin glargine-yfgn'', the first approved insulin biosimilar, which is produced by Viatris, is approved. * 2021: ''Insulin glargine-aglr'', a biosimilar produced by Eli Lilly and Company, is granted approval. * 2024: ''Insulin icodec'', the newest commercially available analogue by Novo Nordisk, gets approval. * 2025: ''Insulin aspart-szjj'', the first short-acting biosimilar, created by Viatris, is approved.Unapproved analogues overview
Many experimental insulin analogues are being developed to improve diabetes treatment. These include new injectable types and oral forms. Oral insulin is being studied as a way to avoid injections and better match natural insulin delivery. * ''Insulin tregopil'', in development by Biocon * ''Insulin efsitora alfa'', made by Lilly'''' * ''ORMD-0801'', an oral analogue produced by Oramed * ''NNC2215'', a glucose-sensitive insulin being researched by Novo Nordisk'''' * ''OI338'' and ''OI320'', two basal oral analogues also by Novo Nordisk'''' * ''LAPS Insulin115'', an ultralong analogue being researched by Hanmi PharmResearch
TheCanadian Agency for Drugs and Technologies in Health
Canada's Drug Agency (CDA; ) is a pan-Canadian health organization responsible for coordinating and aligning drug policy across provinces and territories. The CDA provides Canada's various healthcare organizations with evidence-based advice, allo ...
(CADTH) conducted a 2008 comparison of insulin analogues and biosynthetic human insulin, concluding that insulin analogues did not demonstrate any clinically significant differences in terms of glycemic control or adverse reaction profiles.
Comparative effectiveness
Ameta-analysis
Meta-analysis is a method of synthesis of quantitative data from multiple independent studies addressing a common research question. An important part of this method involves computing a combined effect size across all of the studies. As such, th ...
conducted in 2007 and updated in 2020 by the international Cochrane Collaboration
Cochrane is a British international charitable organisation formed to synthesize medical research findings to facilitate evidence-based choices about health interventions involving health professionals, patients and policy makers. It includes ...
, which reviewed numerous randomized controlled trial
A randomized controlled trial (or randomized control trial; RCT) is a form of scientific experiment used to control factors not under direct experimental control. Examples of RCTs are clinical trials that compare the effects of drugs, surgical ...
s, found that treatment with glargine and detemir insulins resulted in fewer cases of hypoglycemia compared to NPH insulin. Additionally, treatment with detemir was associated with a reduction in the frequency of severe hypoglycemia. However, the review acknowledged limitations, such as the use of low glucose and Hemoglobin A1c
Glycated hemoglobin, also called glycohemoglobin, is a form of hemoglobin (Hb) that is chemically linked to a sugar. Most monosaccharides, including glucose, galactose, and fructose, spontaneously (that is, enzyme, non-enzymatically) bond with h ...
targets, which could affect the generalizability of these findings to routine clinical practice.
In 2007, a report from Germany's Institute for Quality and Cost Effectiveness in the Health Care Sector (IQWiG) concluded that there was insufficient evidence to support the superiority of short-acting insulin analogues over synthetic human insulin for the treatment of adult patients with type 1 diabetes. Many of the studies reviewed were criticized for being too small to provide statistically reliable results, and notably, none were blinded.
See also
* List of commercially available insulinsReferences