Biogeochemical cycle on:
[Wikipedia]
[Google]
[Amazon]
A biogeochemical cycle, or more generally a cycle of matter, is the movement and transformation of chemical elements and compounds between living organisms, the atmosphere, and the Earth's crust. Major biogeochemical cycles include the
 Energy flows directionally through ecosystems, entering as sunlight (or inorganic molecules for chemoautotrophs) and leaving as heat during the many transfers between trophic levels. However, the matter that makes up living organisms is conserved and recycled. The six most common elements associated with organic molecules — carbon, nitrogen, hydrogen, oxygen, phosphorus, and sulfur — take a variety of chemical forms and may exist for long periods in the atmosphere, on land, in water, or beneath the Earth's surface. Geologic processes, such as
Energy flows directionally through ecosystems, entering as sunlight (or inorganic molecules for chemoautotrophs) and leaving as heat during the many transfers between trophic levels. However, the matter that makes up living organisms is conserved and recycled. The six most common elements associated with organic molecules — carbon, nitrogen, hydrogen, oxygen, phosphorus, and sulfur — take a variety of chemical forms and may exist for long periods in the atmosphere, on land, in water, or beneath the Earth's surface. Geologic processes, such as
, ''OpenStax'', 9 May 2019. Material was copied from this source, which is available under
Creative Commons Attribution 4.0 International License
. The six aforementioned elements are used by organisms in a variety of ways. Hydrogen and oxygen are found in water and organic molecules, both of which are essential to life. Carbon is found in all organic molecules, whereas nitrogen is an important component of
3.2 Biogeochemical Cycles
, OpenStax. Material was copied from this source, which is available under
Creative Commons Attribution 4.0 International License
. Ecological systems (
Biogeochemical cycles
. '' Encyclopedia of Earth''. The flow of energy in an ecosystem is an ''open system''; the Sun constantly gives the planet energy in the form of light while it is eventually used and lost in the form of heat throughout the trophic levels of a food web. Carbon is used to make carbohydrates, fats, and proteins, the major sources of
File:BIOGEOCHEMICAL CYCLING OF ELEMENTS.svg, Examples of major biogeochemical processes
File:WhalePump.jpg, The oceanic whale pump showing how whales cycle nutrients through the ocean water column
File:Global carbon cycle.webp, The implications of shifts in the global carbon cycle due to human activity are concerning scientists.
Although the Earth constantly receives energy from the Sun, its chemical composition is essentially fixed, as the additional matter is only occasionally added by meteorites. Because this chemical composition is not replenished like energy, all processes that depend on these chemicals must be recycled. These cycles include both the living biosphere and the nonliving
Creative Commons Attribution 4.0 International License
.

 The global ocean covers more than 70% of the Earth's surface and is remarkably heterogeneous. Marine productive areas, and coastal ecosystems comprise a minor fraction of the ocean in terms of surface area, yet have an enormous impact on global biogeochemical cycles carried out by microbial communities, which represent 90% of the ocean's biomass. Work in recent years has largely focused on cycling of carbon and macronutrients such as nitrogen, phosphorus, and silicate: other important elements such as sulfur or trace elements have been less studied, reflecting associated technical and logistical issues. Increasingly, these marine areas, and the taxa that form their ecosystems, are subject to significant anthropogenic pressure, impacting marine life and recycling of energy and nutrients. A key example is that of cultural eutrophication, where agricultural runoff leads to nitrogen and phosphorus enrichment of coastal ecosystems, greatly increasing productivity resulting in algal blooms, deoxygenation of the water column and seabed, and increased greenhouse gas emissions, with direct local and global impacts on
The global ocean covers more than 70% of the Earth's surface and is remarkably heterogeneous. Marine productive areas, and coastal ecosystems comprise a minor fraction of the ocean in terms of surface area, yet have an enormous impact on global biogeochemical cycles carried out by microbial communities, which represent 90% of the ocean's biomass. Work in recent years has largely focused on cycling of carbon and macronutrients such as nitrogen, phosphorus, and silicate: other important elements such as sulfur or trace elements have been less studied, reflecting associated technical and logistical issues. Increasingly, these marine areas, and the taxa that form their ecosystems, are subject to significant anthropogenic pressure, impacting marine life and recycling of energy and nutrients. A key example is that of cultural eutrophication, where agricultural runoff leads to nitrogen and phosphorus enrichment of coastal ecosystems, greatly increasing productivity resulting in algal blooms, deoxygenation of the water column and seabed, and increased greenhouse gas emissions, with direct local and global impacts on
Creative Commons Attribution 4.0 International License
. Global change is, therefore, affecting key processes including
 Box models are widely used to model biogeochemical systems. Bianchi, Thomas (2007
Box models are widely used to model biogeochemical systems. Bianchi, Thomas (2007
''Biogeochemistry of Estuaries''
page 9, Oxford University Press. . Box models are simplified versions of complex systems, reducing them to boxes (or storage The diagram on the left shows a simplified budget of ocean carbon flows. It is composed of three simple interconnected box models, one for the euphotic zone, one for the ocean interior or dark ocean, and one for ocean sediments. In the euphotic zone, net phytoplankton production is about 50 Pg C each year. About 10 Pg is exported to the ocean interior while the other 40 Pg is respired. Organic carbon degradation occurs as particles ( marine snow) settle through the ocean interior. Only 2 Pg eventually arrives at the seafloor, while the other 8 Pg is respired in the dark ocean. In sediments, the time scale available for degradation increases by orders of magnitude with the result that 90% of the organic carbon delivered is degraded and only 0.2 Pg C yr−1 is eventually buried and transferred from the biosphere to the geosphere.
The diagram on the left shows a simplified budget of ocean carbon flows. It is composed of three simple interconnected box models, one for the euphotic zone, one for the ocean interior or dark ocean, and one for ocean sediments. In the euphotic zone, net phytoplankton production is about 50 Pg C each year. About 10 Pg is exported to the ocean interior while the other 40 Pg is respired. Organic carbon degradation occurs as particles ( marine snow) settle through the ocean interior. Only 2 Pg eventually arrives at the seafloor, while the other 8 Pg is respired in the dark ocean. In sediments, the time scale available for degradation increases by orders of magnitude with the result that 90% of the organic carbon delivered is degraded and only 0.2 Pg C yr−1 is eventually buried and transferred from the biosphere to the geosphere.
 The diagram on the right shows a more complex model with many interacting boxes. Reservoir masses here represents ''carbon stocks'', measured in Pg C. Carbon exchange fluxes, measured in Pg C yr−1, occur between the atmosphere and its two major sinks, the land and the ocean. The black numbers and arrows indicate the reservoir mass and exchange fluxes estimated for the year 1750, just before the
The diagram on the right shows a more complex model with many interacting boxes. Reservoir masses here represents ''carbon stocks'', measured in Pg C. Carbon exchange fluxes, measured in Pg C yr−1, occur between the atmosphere and its two major sinks, the land and the ocean. The black numbers and arrows indicate the reservoir mass and exchange fluxes estimated for the year 1750, just before the
Creative Commons Attribution 4.0 International License
.
 There are fast and slow biogeochemical cycles. Fast cycle operate in the
There are fast and slow biogeochemical cycles. Fast cycle operate in the
Blue planet: The role of the oceans in nutrient cycling, maintain the atmosphere system, and modulating climate change
In: ''Routledge Handbook of Ocean Resources and Management'', Routledge, pages 89–107. . As an example, the fast carbon cycle is illustrated in the diagram on the right. This cycle involves relatively short-term biogeochemical processes between the environment and living organisms in the biosphere. It includes movements of carbon between the atmosphere and terrestrial and marine ecosystems, as well as soils and seafloor sediments. The fast cycle includes annual cycles involving photosynthesis and decadal cycles involving vegetative growth and decomposition. The reactions of the fast carbon cycle to human activities will determine many of the more immediate impacts of climate change. Material was copied from this source, which is available under
Creative Commons Attribution 4.0 International License
. The slow cycle is illustrated in the other diagram. It involves medium to long-term
Creative Commons Attribution 4.0 International License
.
File:Carbon cycle-cute diagram.svg, alt=Diagram of the carbon cycle,
Many biogeochemical cycles are currently being studied for the first time.
File:Plagiomnium affine laminazellen.jpeg, Chloroplasts conduct
Biogeochemical cycles always involve active equilibrium states: a balance in the cycling of the element between compartments. However, overall balance may involve compartments distributed on a global scale.
As biogeochemical cycles describe the movements of substances on the entire globe, the study of these is inherently multidisciplinary. The carbon cycle may be related to research in
DOI 10.1515/9783110589771-002
* * * * {{DEFAULTSORT:Biogeochemical Cycle Biogeography Biosphere Geochemistry
carbon cycle
The carbon cycle is a part of the biogeochemical cycle where carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of Earth. Other major biogeochemical cycles include the nitrogen cycle and the water cycl ...
, the nitrogen cycle
The nitrogen cycle is the biogeochemical cycle by which nitrogen is converted into multiple chemical forms as it circulates among atmosphere, atmospheric, terrestrial ecosystem, terrestrial, and marine ecosystems. The conversion of nitrogen can ...
and the water cycle
The water cycle (or hydrologic cycle or hydrological cycle) is a biogeochemical cycle that involves the continuous movement of water on, above and below the surface of the Earth across different reservoirs. The mass of water on Earth remains fai ...
. In each cycle, the chemical element or molecule is transformed and cycled by living organisms and through various geological forms and reservoirs, including the atmosphere, the soil and the oceans. It can be thought of as the pathway by which a chemical substance
A chemical substance is a unique form of matter with constant chemical composition and characteristic properties. Chemical substances may take the form of a single element or chemical compounds. If two or more chemical substances can be com ...
cycles (is turned over or moves through) the biotic compartment and the abiotic compartments of Earth
Earth is the third planet from the Sun and the only astronomical object known to Planetary habitability, harbor life. This is enabled by Earth being an ocean world, the only one in the Solar System sustaining liquid surface water. Almost all ...
. The biotic compartment is the biosphere
The biosphere (), also called the ecosphere (), is the worldwide sum of all ecosystems. It can also be termed the zone of life on the Earth. The biosphere (which is technically a spherical shell) is virtually a closed system with regard to mat ...
and the abiotic compartments are the atmosphere
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosph ...
, lithosphere
A lithosphere () is the rigid, outermost rocky shell of a terrestrial planet or natural satellite. On Earth, it is composed of the crust and the lithospheric mantle, the topmost portion of the upper mantle that behaves elastically on time ...
and hydrosphere
The hydrosphere () is the combined mass of water found on, under, and above the Planetary surface, surface of a planet, minor planet, or natural satellite. Although Earth's hydrosphere has been around for about 4 billion years, it continues to ch ...
.
For example, in the carbon cycle, atmospheric carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
is absorbed by plants through photosynthesis
Photosynthesis ( ) is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabo ...
, which converts it into organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
s that are used by organisms for energy and growth. Carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
is then released back into the atmosphere through respiration and decomposition
Decomposition is the process by which dead organic substances are broken down into simpler organic or inorganic matter such as carbon dioxide, water, simple sugars and mineral salts. The process is a part of the nutrient cycle and is ess ...
. Additionally, carbon is stored in fossil fuel
A fossil fuel is a flammable carbon compound- or hydrocarbon-containing material formed naturally in the Earth's crust from the buried remains of prehistoric organisms (animals, plants or microplanktons), a process that occurs within geolog ...
s and is released into the atmosphere through human activities such as burning fossil fuels
A fossil fuel is a flammable carbon compound- or hydrocarbon-containing material formed naturally in the Earth's crust from the buried remains of prehistoric organisms (animals, plants or microplanktons), a process that occurs within geologica ...
. In the nitrogen cycle, atmospheric nitrogen gas is converted by plants into usable forms such as ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
and nitrate
Nitrate is a polyatomic ion with the chemical formula . salt (chemistry), Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are solubility, soluble in wa ...
s through the process of nitrogen fixation
Nitrogen fixation is a chemical process by which molecular dinitrogen () is converted into ammonia (). It occurs both biologically and abiological nitrogen fixation, abiologically in chemical industry, chemical industries. Biological nitrogen ...
. These compounds can be used by other organisms, and nitrogen is returned to the atmosphere through denitrification and other processes. In the water cycle, the universal solvent water evaporates from land and oceans to form clouds in the atmosphere, and then precipitates back to different parts of the planet. Precipitation can seep into the ground and become part of groundwater systems used by plants and other organisms, or can runoff the surface to form lakes and rivers. Subterranean water can then seep into the ocean along with river discharges, rich with dissolved and particulate organic matter and other nutrients.
There are biogeochemical cycles for many other elements, such as for oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
, hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
, phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ...
, calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to it ...
, iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
, sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
, mercury and selenium
Selenium is a chemical element; it has symbol (chemistry), symbol Se and atomic number 34. It has various physical appearances, including a brick-red powder, a vitreous black solid, and a grey metallic-looking form. It seldom occurs in this elem ...
. There are also cycles for molecules, such as water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
and silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant f ...
. In addition there are macroscopic cycles such as the rock cycle
The ''rock cycle'' is a basic concept in geology that describes transitions through geologic time among the three main rock types: sedimentary, metamorphic, and igneous. Each rock type is altered when it is forced out of its equilibrium cond ...
, and human-induced cycles for synthetic compounds such as for polychlorinated biphenyls (PCBs). In some cycles there are geological reservoirs where substances can remain or be sequestered for long periods of time.
Biogeochemical cycles involve the interaction of biological, geological, and chemical processes. Biological processes include the influence of microorganism
A microorganism, or microbe, is an organism of microscopic scale, microscopic size, which may exist in its unicellular organism, single-celled form or as a Colony (biology)#Microbial colonies, colony of cells. The possible existence of unseen ...
s, which are critical drivers of biogeochemical cycling. Microorganisms have the ability to carry out wide ranges of metabolic processes essential for the cycling of nutrients ( macronutrients and micronutrients) and chemicals throughout global ecosystems. Without microorganisms many of these processes would not occur, with significant impact on the functioning of land and ocean ecosystems and the planet's biogeochemical cycles as a whole. Changes to cycles can impact human health. The cycles are interconnected and play important roles regulating climate, supporting the growth of plant
Plants are the eukaryotes that form the Kingdom (biology), kingdom Plantae; they are predominantly Photosynthesis, photosynthetic. This means that they obtain their energy from sunlight, using chloroplasts derived from endosymbiosis with c ...
s, phytoplankton and other organisms, and maintaining the health of ecosystems generally. Human activities such as burning fossil fuels and using large amounts of fertilizer can disrupt cycles, contributing to climate change, pollution, and other environmental problems.
Overview
 Energy flows directionally through ecosystems, entering as sunlight (or inorganic molecules for chemoautotrophs) and leaving as heat during the many transfers between trophic levels. However, the matter that makes up living organisms is conserved and recycled. The six most common elements associated with organic molecules — carbon, nitrogen, hydrogen, oxygen, phosphorus, and sulfur — take a variety of chemical forms and may exist for long periods in the atmosphere, on land, in water, or beneath the Earth's surface. Geologic processes, such as
Energy flows directionally through ecosystems, entering as sunlight (or inorganic molecules for chemoautotrophs) and leaving as heat during the many transfers between trophic levels. However, the matter that makes up living organisms is conserved and recycled. The six most common elements associated with organic molecules — carbon, nitrogen, hydrogen, oxygen, phosphorus, and sulfur — take a variety of chemical forms and may exist for long periods in the atmosphere, on land, in water, or beneath the Earth's surface. Geologic processes, such as weathering
Weathering is the deterioration of rocks, soils and minerals (as well as wood and artificial materials) through contact with water, atmospheric gases, sunlight, and biological organisms. It occurs '' in situ'' (on-site, with little or no move ...
, erosion
Erosion is the action of surface processes (such as Surface runoff, water flow or wind) that removes soil, Rock (geology), rock, or dissolved material from one location on the Earth's crust#Crust, Earth's crust and then sediment transport, tran ...
, water drainage, and the subduction of the continental plates, all play a role in this recycling of materials. Because geology
Geology (). is a branch of natural science concerned with the Earth and other astronomical objects, the rocks of which they are composed, and the processes by which they change over time. Modern geology significantly overlaps all other Earth ...
and chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
have major roles in the study of this process, the recycling of inorganic matter between living organisms and their environment is called a biogeochemical cycle.Biogeochemical Cycles, ''OpenStax'', 9 May 2019. Material was copied from this source, which is available under
Creative Commons Attribution 4.0 International License
. The six aforementioned elements are used by organisms in a variety of ways. Hydrogen and oxygen are found in water and organic molecules, both of which are essential to life. Carbon is found in all organic molecules, whereas nitrogen is an important component of
nucleic acid
Nucleic acids are large biomolecules that are crucial in all cells and viruses. They are composed of nucleotides, which are the monomer components: a pentose, 5-carbon sugar, a phosphate group and a nitrogenous base. The two main classes of nuclei ...
s and protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
s. Phosphorus is used to make nucleic acids and the phospholipid
Phospholipids are a class of lipids whose molecule has a hydrophilic "head" containing a phosphate group and two hydrophobic "tails" derived from fatty acids, joined by an alcohol residue (usually a glycerol molecule). Marine phospholipids typ ...
s that comprise biological membranes. Sulfur is critical to the three-dimensional shape of proteins. The cycling of these elements is interconnected. For example, the movement of water is critical for leaching sulfur and phosphorus into rivers which can then flow into oceans. Minerals cycle through the biosphere between the biotic and abiotic components and from one organism to another.Fisher M. R. (Ed.) (2019) ''Environmental Biology''3.2 Biogeochemical Cycles
, OpenStax. Material was copied from this source, which is available under
Creative Commons Attribution 4.0 International License
. Ecological systems (
ecosystem
An ecosystem (or ecological system) is a system formed by Organism, organisms in interaction with their Biophysical environment, environment. The Biotic material, biotic and abiotic components are linked together through nutrient cycles and en ...
s) have many biogeochemical cycles operating as a part of the system, for example, the water cycle, the carbon cycle, the nitrogen cycle, etc. All chemical elements occurring in organisms are part of biogeochemical cycles. In addition to being a part of living organisms, these chemical elements also cycle through abiotic factors of ecosystems such as water (hydrosphere
The hydrosphere () is the combined mass of water found on, under, and above the Planetary surface, surface of a planet, minor planet, or natural satellite. Although Earth's hydrosphere has been around for about 4 billion years, it continues to ch ...
), land (lithosphere
A lithosphere () is the rigid, outermost rocky shell of a terrestrial planet or natural satellite. On Earth, it is composed of the crust and the lithospheric mantle, the topmost portion of the upper mantle that behaves elastically on time ...
), and/or the air (atmosphere
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosph ...
).
The living factors of the planet can be referred to collectively as the biosphere
The biosphere (), also called the ecosphere (), is the worldwide sum of all ecosystems. It can also be termed the zone of life on the Earth. The biosphere (which is technically a spherical shell) is virtually a closed system with regard to mat ...
. All the nutrients — such as carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
, nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
, oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
, phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ...
, and sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
— used in ecosystems by living organisms are a part of a ''closed system''; therefore, these chemicals are recycled instead of being lost and replenished constantly such as in an open system.
The major parts of the biosphere are connected by the flow of chemical elements and compounds in biogeochemical cycles. In many of these cycles, the biota plays an important role. Matter from the Earth's interior is released by volcanoes. The atmosphere exchanges some compounds and elements rapidly with the biota and oceans. Exchanges of materials between rocks, soils, and the oceans are generally slower by comparison.Moses, M. (2012Biogeochemical cycles
. '' Encyclopedia of Earth''. The flow of energy in an ecosystem is an ''open system''; the Sun constantly gives the planet energy in the form of light while it is eventually used and lost in the form of heat throughout the trophic levels of a food web. Carbon is used to make carbohydrates, fats, and proteins, the major sources of
food energy
Food energy is chemical energy that animals and humans derive from food to sustain their metabolism and muscular activity.
Most animals derive most of their energy from aerobic respiration, namely combining the carbohydrates, fats, and protein ...
. These compounds are oxidized to release carbon dioxide, which can be captured by plants to make organic compounds. The chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
is powered by the light energy of sunshine.
Sunlight is required to combine carbon with hydrogen and oxygen into an energy source, but ecosystems in the deep sea, where no sunlight can penetrate, obtain energy from sulfur. Hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist ...
near hydrothermal vents can be utilized by organisms such as the giant tube worm. In the sulfur cycle
The sulfur cycle is a biogeochemical cycle in which the sulfur moves between rocks, waterways and living systems. It is important in geology as it affects many minerals and in life because sulfur is an essential element (CHNOPS), being a consti ...
, sulfur can be forever recycled as a source of energy. Energy can be released through the oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
and reduction of sulfur compounds (e.g., oxidizing elemental sulfur to sulfite and then to sulfate).
lithosphere
A lithosphere () is the rigid, outermost rocky shell of a terrestrial planet or natural satellite. On Earth, it is composed of the crust and the lithospheric mantle, the topmost portion of the upper mantle that behaves elastically on time ...
, atmosphere
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosph ...
, and hydrosphere
The hydrosphere () is the combined mass of water found on, under, and above the Planetary surface, surface of a planet, minor planet, or natural satellite. Although Earth's hydrosphere has been around for about 4 billion years, it continues to ch ...
.
Biogeochemical cycles can be contrasted with geochemical cycles. The latter deals only with crustal and subcrustal reservoirs even though some process from both overlap.
Compartments
Bogeochemical cycles operate by moving substances, which may also undergo chemical rearrangments, through pathways in the biotic compartment and the abiotic compartments of Earth. The biotic compartment is thebiosphere
The biosphere (), also called the ecosphere (), is the worldwide sum of all ecosystems. It can also be termed the zone of life on the Earth. The biosphere (which is technically a spherical shell) is virtually a closed system with regard to mat ...
and the abiotic compartments are the atmosphere
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosph ...
, lithosphere
A lithosphere () is the rigid, outermost rocky shell of a terrestrial planet or natural satellite. On Earth, it is composed of the crust and the lithospheric mantle, the topmost portion of the upper mantle that behaves elastically on time ...
and hydrosphere
The hydrosphere () is the combined mass of water found on, under, and above the Planetary surface, surface of a planet, minor planet, or natural satellite. Although Earth's hydrosphere has been around for about 4 billion years, it continues to ch ...
.
Biotic compartment
Biosphere
Microorganisms drive much of the biogeochemical cycling in the earth system. Material was copied from this source, which is available underCreative Commons Attribution 4.0 International License
.
Abiotic compartments

Atmosphere
Hydrosphere
 The global ocean covers more than 70% of the Earth's surface and is remarkably heterogeneous. Marine productive areas, and coastal ecosystems comprise a minor fraction of the ocean in terms of surface area, yet have an enormous impact on global biogeochemical cycles carried out by microbial communities, which represent 90% of the ocean's biomass. Work in recent years has largely focused on cycling of carbon and macronutrients such as nitrogen, phosphorus, and silicate: other important elements such as sulfur or trace elements have been less studied, reflecting associated technical and logistical issues. Increasingly, these marine areas, and the taxa that form their ecosystems, are subject to significant anthropogenic pressure, impacting marine life and recycling of energy and nutrients. A key example is that of cultural eutrophication, where agricultural runoff leads to nitrogen and phosphorus enrichment of coastal ecosystems, greatly increasing productivity resulting in algal blooms, deoxygenation of the water column and seabed, and increased greenhouse gas emissions, with direct local and global impacts on
The global ocean covers more than 70% of the Earth's surface and is remarkably heterogeneous. Marine productive areas, and coastal ecosystems comprise a minor fraction of the ocean in terms of surface area, yet have an enormous impact on global biogeochemical cycles carried out by microbial communities, which represent 90% of the ocean's biomass. Work in recent years has largely focused on cycling of carbon and macronutrients such as nitrogen, phosphorus, and silicate: other important elements such as sulfur or trace elements have been less studied, reflecting associated technical and logistical issues. Increasingly, these marine areas, and the taxa that form their ecosystems, are subject to significant anthropogenic pressure, impacting marine life and recycling of energy and nutrients. A key example is that of cultural eutrophication, where agricultural runoff leads to nitrogen and phosphorus enrichment of coastal ecosystems, greatly increasing productivity resulting in algal blooms, deoxygenation of the water column and seabed, and increased greenhouse gas emissions, with direct local and global impacts on nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
and carbon cycle
The carbon cycle is a part of the biogeochemical cycle where carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of Earth. Other major biogeochemical cycles include the nitrogen cycle and the water cycl ...
s. However, the runoff of organic matter
Organic matter, organic material or natural organic matter is the large source of carbon-based compounds found within natural and engineered, terrestrial, and aquatic environments. It is matter composed of organic compounds that have come fro ...
from the mainland to coastal ecosystems is just one of a series of pressing threats stressing microbial communities due to global change. Climate change has also resulted in changes in the cryosphere, as glaciers and permafrost melt, resulting in intensified marine stratification, while shifts of the redox-state in different biomes are rapidly reshaping microbial assemblages at an unprecedented rate. Material was copied from this source, which is available under Creative Commons Attribution 4.0 International License
. Global change is, therefore, affecting key processes including
primary productivity
Primary or primaries may refer to:
Arts, entertainment, and media Music Groups and labels
* Primary (band), from Australia
* Primary (musician), hip hop musician and record producer from South Korea
* Primary Music, Israeli record label
Works
* ...
, CO2 and N2 fixation, organic matter respiration/ remineralization, and the sinking and burial deposition of fixed CO2. In addition to this, oceans are experiencing an acidification process, with a change of ~0.1 pH units between the pre-industrial period and today, affecting carbonate
A carbonate is a salt of carbonic acid, (), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate group ...
/bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial bioche ...
buffer chemistry. In turn, acidification has been reported to impact planktonic
Plankton are the diverse collection of organisms that drift in water (or air) but are unable to actively propel themselves against currents (or wind). The individual organisms constituting plankton are called plankters. In the ocean, they pro ...
communities, principally through effects on calcifying taxa. There is also evidence for shifts in the production of key intermediary volatile products, some of which have marked greenhouse effects (e.g., N2O and CH4, reviewed by Breitburg in 2018, due to the increase in global temperature, ocean stratification and deoxygenation, driving as much as 25 to 50% of nitrogen loss from the ocean to the atmosphere in the so-called oxygen minimum zones or anoxic marine zones, driven by microbial processes. Other products, that are typically toxic for the marine nekton, including reduced sulfur species such as H2S, have a negative impact for marine resources like fisheries and coastal aquaculture. While global change has accelerated, there has been a parallel increase in awareness of the complexity of marine ecosystems, and especially the fundamental role of microbes as drivers of ecosystem functioning.
Lithosphere
Reservoirs
The chemicals are sometimes held for long periods of time in one place. This place is called a ''reservoir'', which, for example, includes such things ascoal
Coal is a combustible black or brownish-black sedimentary rock, formed as rock strata called coal seams. Coal is mostly carbon with variable amounts of other Chemical element, elements, chiefly hydrogen, sulfur, oxygen, and nitrogen.
Coal i ...
deposits that are storing carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
for a long period of time. When chemicals are held for only short periods of time, they are being held in ''exchange pools''. Examples of exchange pools include plants and animals.
Plants and animals utilize carbon to produce carbohydrates, fats, and proteins, which can then be used to build their internal structures or to obtain energy. Plants and animals temporarily use carbon in their systems and then release it back into the air or surrounding medium. Generally, reservoirs are abiotic factors whereas exchange pools are biotic factors. Carbon is held for a relatively short time in plants and animals in comparison to coal deposits. The amount of time that a chemical is held in one place is called its residence time or turnover time (also called the renewal time or exit age).
Box models
 Box models are widely used to model biogeochemical systems. Bianchi, Thomas (2007
Box models are widely used to model biogeochemical systems. Bianchi, Thomas (2007''Biogeochemistry of Estuaries''
page 9, Oxford University Press. . Box models are simplified versions of complex systems, reducing them to boxes (or storage
reservoir
A reservoir (; ) is an enlarged lake behind a dam, usually built to water storage, store fresh water, often doubling for hydroelectric power generation.
Reservoirs are created by controlling a watercourse that drains an existing body of wa ...
s) for chemical materials, linked by material fluxes (flows). Simple box models have a small number of boxes with properties, such as volume, that do not change with time. The boxes are assumed to behave as if they were mixed homogeneously. These models are often used to derive analytical formulas describing the dynamics and steady-state abundance of the chemical species involved.
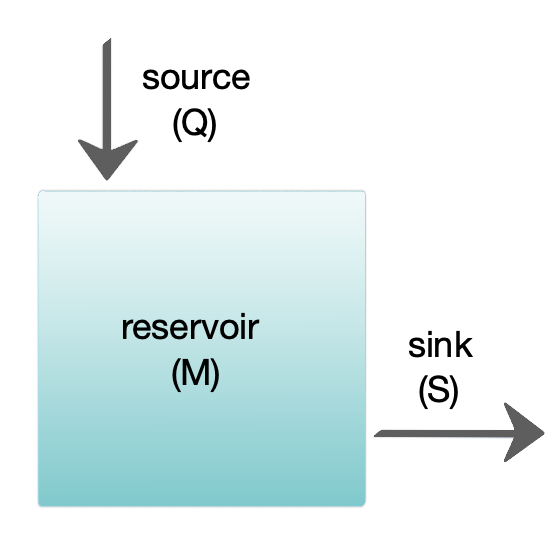
The diagram at the right shows a basic one-box model. The reservoir contains the amount of material ''M'' under consideration, as defined by chemical, physical or biological properties. The source ''Q'' is the flux of material into the reservoir, and the sink ''S'' is the flux of material out of the reservoir. The budget is the check and balance of the sources and sinks affecting material turnover in a reservoir. The reservoir is in a steady state if ''Q'' = ''S'', that is, if the sources balance the sinks and there is no change over time.
The residence or turnover time is the average time material spends resident in the reservoir. If the reservoir is in a steady state, this is the same as the time it takes to fill or drain the reservoir. Thus, if τ is the turnover time, then τ = ''M''/''S''. The equation describing the rate of change of content in a reservoir is
:
When two or more reservoirs are connected, the material can be regarded as cycling between the reservoirs, and there can be predictable patterns to the cyclic flow. More complex multibox models are usually solved using numerical techniques.
 The diagram on the left shows a simplified budget of ocean carbon flows. It is composed of three simple interconnected box models, one for the euphotic zone, one for the ocean interior or dark ocean, and one for ocean sediments. In the euphotic zone, net phytoplankton production is about 50 Pg C each year. About 10 Pg is exported to the ocean interior while the other 40 Pg is respired. Organic carbon degradation occurs as particles ( marine snow) settle through the ocean interior. Only 2 Pg eventually arrives at the seafloor, while the other 8 Pg is respired in the dark ocean. In sediments, the time scale available for degradation increases by orders of magnitude with the result that 90% of the organic carbon delivered is degraded and only 0.2 Pg C yr−1 is eventually buried and transferred from the biosphere to the geosphere.
The diagram on the left shows a simplified budget of ocean carbon flows. It is composed of three simple interconnected box models, one for the euphotic zone, one for the ocean interior or dark ocean, and one for ocean sediments. In the euphotic zone, net phytoplankton production is about 50 Pg C each year. About 10 Pg is exported to the ocean interior while the other 40 Pg is respired. Organic carbon degradation occurs as particles ( marine snow) settle through the ocean interior. Only 2 Pg eventually arrives at the seafloor, while the other 8 Pg is respired in the dark ocean. In sediments, the time scale available for degradation increases by orders of magnitude with the result that 90% of the organic carbon delivered is degraded and only 0.2 Pg C yr−1 is eventually buried and transferred from the biosphere to the geosphere.
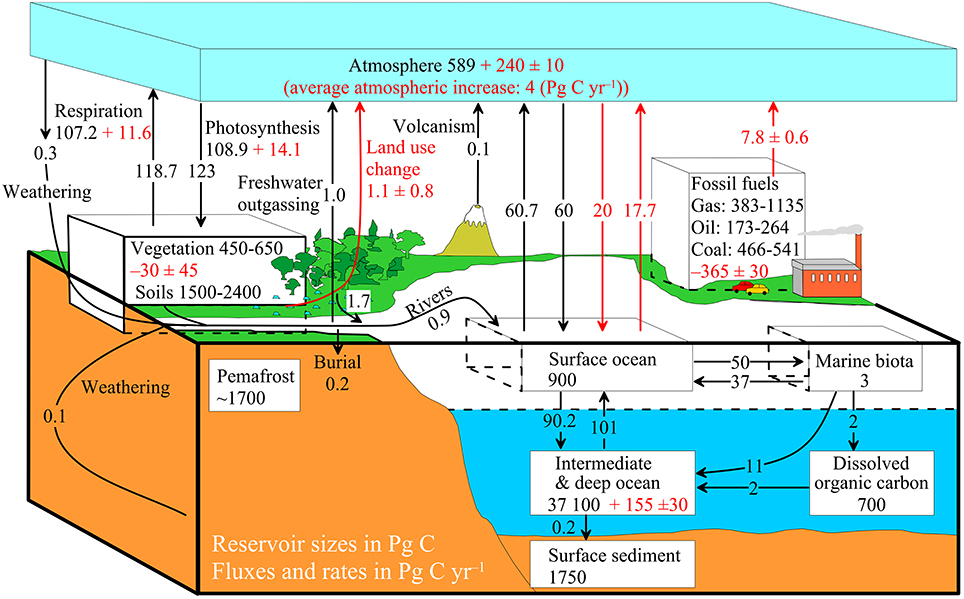
 The diagram on the right shows a more complex model with many interacting boxes. Reservoir masses here represents ''carbon stocks'', measured in Pg C. Carbon exchange fluxes, measured in Pg C yr−1, occur between the atmosphere and its two major sinks, the land and the ocean. The black numbers and arrows indicate the reservoir mass and exchange fluxes estimated for the year 1750, just before the
The diagram on the right shows a more complex model with many interacting boxes. Reservoir masses here represents ''carbon stocks'', measured in Pg C. Carbon exchange fluxes, measured in Pg C yr−1, occur between the atmosphere and its two major sinks, the land and the ocean. The black numbers and arrows indicate the reservoir mass and exchange fluxes estimated for the year 1750, just before the Industrial Revolution
The Industrial Revolution, sometimes divided into the First Industrial Revolution and Second Industrial Revolution, was a transitional period of the global economy toward more widespread, efficient and stable manufacturing processes, succee ...
. The red arrows (and associated numbers) indicate the annual flux changes due to anthropogenic activities, averaged over the 2000–2009 time period. They represent how the carbon cycle has changed since 1750. Red numbers in the reservoirs represent the cumulative changes in anthropogenic carbon since the start of the Industrial Period, 1750–2011. Material was copied from this source, which is available under Creative Commons Attribution 4.0 International License
.
Fast and slow cycles
 There are fast and slow biogeochemical cycles. Fast cycle operate in the
There are fast and slow biogeochemical cycles. Fast cycle operate in the biosphere
The biosphere (), also called the ecosphere (), is the worldwide sum of all ecosystems. It can also be termed the zone of life on the Earth. The biosphere (which is technically a spherical shell) is virtually a closed system with regard to mat ...
and slow cycles operate in the lithosphere
A lithosphere () is the rigid, outermost rocky shell of a terrestrial planet or natural satellite. On Earth, it is composed of the crust and the lithospheric mantle, the topmost portion of the upper mantle that behaves elastically on time ...
in rocks. Fast or biological cycles can complete within years, moving substances from atmosphere to biosphere, then back to the atmosphere. Slow or geological cycles can take millions of years to complete, moving substances through the Earth's crust between rocks, soil, ocean and atmosphere.Libes, Susan M. (2015)Blue planet: The role of the oceans in nutrient cycling, maintain the atmosphere system, and modulating climate change
In: ''Routledge Handbook of Ocean Resources and Management'', Routledge, pages 89–107. . As an example, the fast carbon cycle is illustrated in the diagram on the right. This cycle involves relatively short-term biogeochemical processes between the environment and living organisms in the biosphere. It includes movements of carbon between the atmosphere and terrestrial and marine ecosystems, as well as soils and seafloor sediments. The fast cycle includes annual cycles involving photosynthesis and decadal cycles involving vegetative growth and decomposition. The reactions of the fast carbon cycle to human activities will determine many of the more immediate impacts of climate change. Material was copied from this source, which is available under
Creative Commons Attribution 4.0 International License
. The slow cycle is illustrated in the other diagram. It involves medium to long-term
geochemical
Geochemistry is the science that uses the tools and principles of chemistry to explain the mechanisms behind major geological systems such as the Earth's crust and its oceans. The realm of geochemistry extends beyond the Earth, encompassing the ...
processes belonging to the rock cycle
The ''rock cycle'' is a basic concept in geology that describes transitions through geologic time among the three main rock types: sedimentary, metamorphic, and igneous. Each rock type is altered when it is forced out of its equilibrium cond ...
. The exchange between the ocean and atmosphere can take centuries, and the weathering
Weathering is the deterioration of rocks, soils and minerals (as well as wood and artificial materials) through contact with water, atmospheric gases, sunlight, and biological organisms. It occurs '' in situ'' (on-site, with little or no move ...
of rocks can take millions of years. Carbon in the ocean precipitates to the ocean floor where it can form sedimentary rock
Sedimentary rocks are types of rock (geology), rock formed by the cementation (geology), cementation of sediments—i.e. particles made of minerals (geological detritus) or organic matter (biological detritus)—that have been accumulated or de ...
and be subducted into the Earth's mantle. Mountain building processes result in the return of this geologic carbon to the Earth's surface. There the rocks are weathered and carbon is returned to the atmosphere by degassing and to the ocean by rivers. Other geologic carbon returns to the ocean through the hydrothermal emission of calcium ions. In a given year between 10 and 100 million tonnes of carbon moves around this slow cycle. This includes volcanoes returning geologic carbon directly to the atmosphere in the form of carbon dioxide. However, this is less than one percent of the carbon dioxide put into the atmosphere by burning fossil fuels.
Deep cycles
The terrestrial subsurface is the largest reservoir of carbon on earth, containing 14–135 Pg of carbon and 2–19% of all biomass. Microorganisms drive organic and inorganic compound transformations in this environment and thereby control biogeochemical cycles. Current knowledge of the microbial ecology of the subsurface is primarily based on16S ribosomal RNA
16S ribosomal RNA (or 16 S rRNA) is the RNA component of the 30S subunit of a prokaryotic ribosome ( SSU rRNA). It binds to the Shine-Dalgarno sequence and provides most of the SSU structure.
The genes coding for it are referred to as 16S ...
(rRNA) gene sequences. Recent estimates show that <8% of 16S rRNA sequences in public databases derive from subsurface organisms and only a small fraction of those are represented by genomes or isolates. Thus, there is remarkably little reliable information about microbial metabolism in the subsurface. Further, little is known about how organisms in subsurface ecosystems are metabolically interconnected. Some cultivation-based studies of syntrophic consortia and small-scale metagenomic analyses of natural communities suggest that organisms are linked via metabolic handoffs: the transfer of redox reaction products of one organism to another. However, no complex environments have been dissected completely enough to resolve the metabolic interaction networks that underpin them. This restricts the ability of biogeochemical models to capture key aspects of the carbon and other nutrient cycles. New approaches such as genome-resolved metagenomics, an approach that can yield a comprehensive set of draft and even complete genomes for organisms without the requirement for laboratory isolation have the potential to provide this critical level of understanding of biogeochemical processes. Material was copied from this source, which is available under Creative Commons Attribution 4.0 International License
.
Some examples
Some of the more well-known biogeochemical cycles are shown below:Carbon cycle
The carbon cycle is a part of the biogeochemical cycle where carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of Earth. Other major biogeochemical cycles include the nitrogen cycle and the water cycl ...
File:Oxygen Cycle.jpg, Oxygen cycle
File:Nitrogen_Cycle.jpg, alt=Diagram of the nitrogen cycle, Nitrogen cycle
The nitrogen cycle is the biogeochemical cycle by which nitrogen is converted into multiple chemical forms as it circulates among atmosphere, atmospheric, terrestrial ecosystem, terrestrial, and marine ecosystems. The conversion of nitrogen can ...
File:WhalePump.jpg, alt=Diagram of the nutrient cycle, Nutrient cycle
A nutrient cycle (or ecological recycling) is the movement and exchange of inorganic and organic matter back into the production of matter. Energy flow is a unidirectional and noncyclic pathway, whereas the movement of mineral nutrients is cyc ...
File:Phosphorus cycle.png, alt=Diagram of the phosphorus cycle, Phosphorus cycle
The phosphorus cycle is the biogeochemical cycle that involves the movement of phosphorus through the lithosphere, hydrosphere, and biosphere. Unlike many other biogeochemical cycles, the atmosphere does not play a significant role in the moveme ...
File:Sulfur Cycle (Ciclo do Enxofre).png, alt=Diagram of the sulfur cycle, Sulfur cycle
The sulfur cycle is a biogeochemical cycle in which the sulfur moves between rocks, waterways and living systems. It is important in geology as it affects many minerals and in life because sulfur is an essential element (CHNOPS), being a consti ...
File:Cycle of rocks 1.png, alt=Diagram of the rock cycle, Rock cycle
The ''rock cycle'' is a basic concept in geology that describes transitions through geologic time among the three main rock types: sedimentary, metamorphic, and igneous. Each rock type is altered when it is forced out of its equilibrium cond ...
File:Water cycle.png, alt=Diagram of the water cycle, Water cycle
The water cycle (or hydrologic cycle or hydrological cycle) is a biogeochemical cycle that involves the continuous movement of water on, above and below the surface of the Earth across different reservoirs. The mass of water on Earth remains fai ...
Climate change
Present-day climate change includes both global warming—the ongoing increase in Global surface temperature, global average temperature—and its wider effects on Earth's climate system. Climate variability and change, Climate change in ...
and human impacts are drastically changing the speed, intensity, and balance of these relatively unknown cycles, which include:
* the mercury cycle, and
* the human-caused cycle of PCBs.
photosynthesis
Photosynthesis ( ) is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabo ...
in plant cell
Plant cells are the cells present in Viridiplantae, green plants, photosynthetic eukaryotes of the kingdom Plantae. Their distinctive features include primary cell walls containing cellulose, hemicelluloses and pectin, the presence of plastids ...
s and other eukaryotic
The eukaryotes ( ) constitute the Domain (biology), domain of Eukaryota or Eukarya, organisms whose Cell (biology), cells have a membrane-bound cell nucleus, nucleus. All animals, plants, Fungus, fungi, seaweeds, and many unicellular organisms ...
organisms.
File:Organic carbon cycle including the flow of kerogen.png, Kerogen cycle
File:Coal anthracite.jpg, Coal is a reservoir of carbon
ecology
Ecology () is the natural science of the relationships among living organisms and their Natural environment, environment. Ecology considers organisms at the individual, population, community (ecology), community, ecosystem, and biosphere lev ...
and atmospheric sciences. Biochemical dynamics would also be related to the fields of geology
Geology (). is a branch of natural science concerned with the Earth and other astronomical objects, the rocks of which they are composed, and the processes by which they change over time. Modern geology significantly overlaps all other Earth ...
and pedology.
See also
* * * * *References
Further reading
*Schink, Bernhard; "Microbes: Masters of the Global Element Cycles" pp 33–58. "Metals, Microbes and Minerals: The Biogeochemical Side of Life", pp xiv + 341. Walter de Gruyter, BerlinDOI 10.1515/9783110589771-002
* * * * {{DEFAULTSORT:Biogeochemical Cycle Biogeography Biosphere Geochemistry