Ocean acidification is the ongoing decrease in the
pH of the Earth's
ocean
The ocean is the body of salt water that covers approximately 70.8% of Earth. The ocean is conventionally divided into large bodies of water, which are also referred to as ''oceans'' (the Pacific, Atlantic, Indian Ocean, Indian, Southern Ocean ...
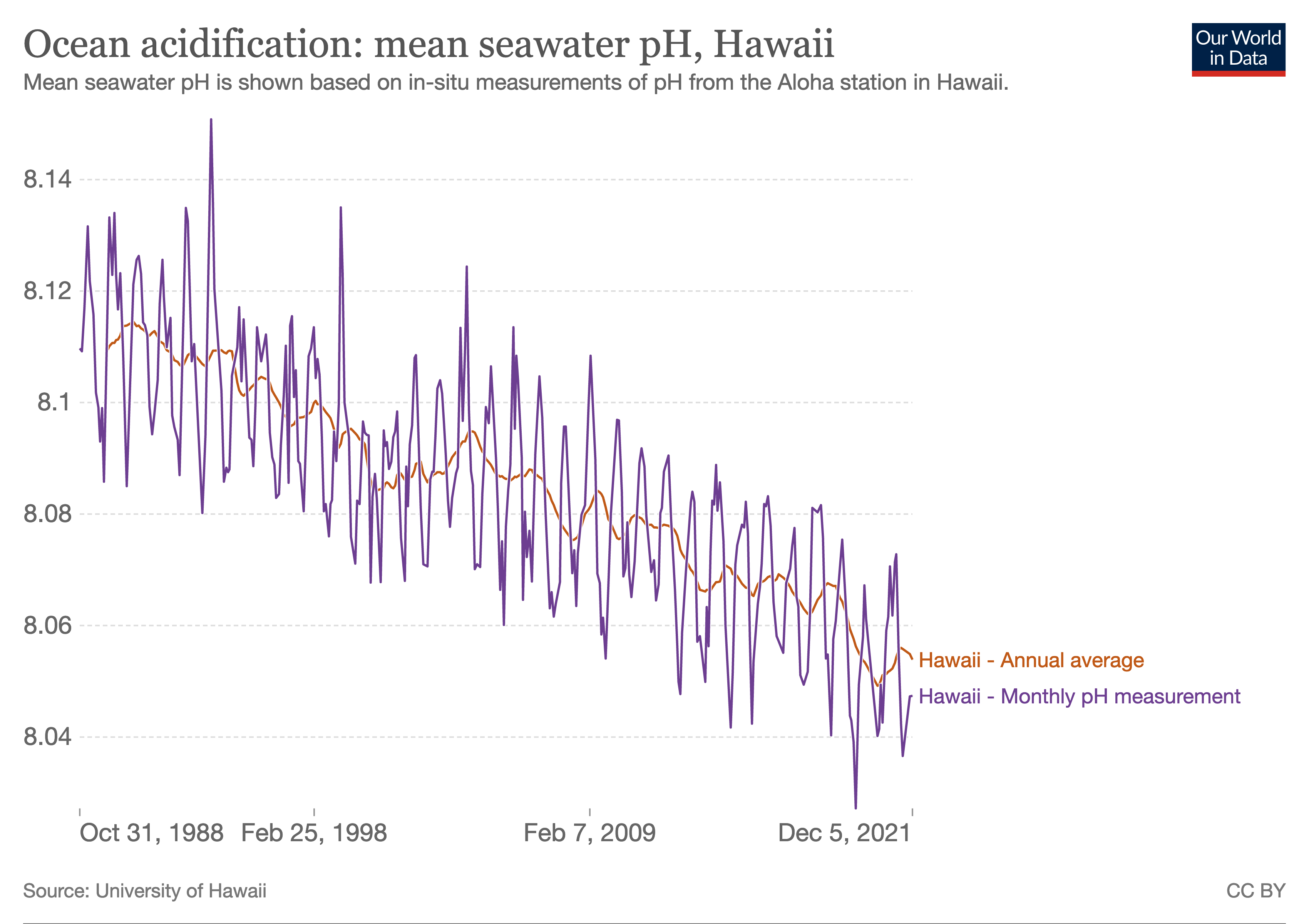
. Between 1950 and 2020, the average pH of the ocean surface fell from approximately 8.15 to 8.05.
Carbon dioxide emissions
Greenhouse gas (GHG) emissions from human activities intensify the greenhouse effect. This contributes to climate change. Carbon dioxide (), from burning fossil fuels such as coal, oil, and natural gas, is the main cause of climate change. The ...
from human activities are the primary cause of ocean acidification, with
atmospheric carbon dioxide () levels exceeding 422 ppm (). from the
atmosphere
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosph ...
is absorbed by the oceans. This chemical reaction produces
carbonic acid
Carbonic acid is a chemical compound with the chemical formula . The molecule rapidly converts to water and carbon dioxide in the presence of water. However, in the absence of water, it is quite stable at room temperature. The interconversion ...
() which
dissociate
Dissociation in chemistry is a general process in which molecules (or ionic compounds such as salts, or complexes) separate or split into other things such as atoms, ions, or radicals, usually in a reversible manner. For instance, when an aci ...
s into a
bicarbonate ion
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial biochemical ...
() and a
hydrogen ion
A hydrogen ion is created when a hydrogen atom loses or gains an electron. A positively charged hydrogen ion (or proton) can readily combine with other particles and therefore is only seen isolated when it is in a gaseous state or a nearly particl ...
(). The presence of free hydrogen ions () lowers the pH of the ocean, increasing
acid
An acid is a molecule or ion capable of either donating a proton (i.e. Hydron, hydrogen cation, H+), known as a Brønsted–Lowry acid–base theory, Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis ...
ity (this does not mean that
seawater
Seawater, or sea water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approximat ...
is acidic yet; it is still
alkali
In chemistry, an alkali (; from the Arabic word , ) is a basic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a soluble base has a pH greater than 7.0. The a ...
ne, with a pH higher than 8).
Marine calcifying organisms, such as
mollusks
Mollusca is a phylum of protostomic invertebrate animals, whose members are known as molluscs or mollusks (). Around 76,000 extant species of molluscs are recognized, making it the second-largest animal phylum after Arthropoda. The num ...
and
coral
Corals are colonial marine invertebrates within the subphylum Anthozoa of the phylum Cnidaria. They typically form compact Colony (biology), colonies of many identical individual polyp (zoology), polyps. Coral species include the important Coral ...
s, are especially vulnerable because they rely on calcium carbonate to build shells and skeletons.
A change in pH by 0.1 represents a 26% increase in hydrogen ion concentration in the world's oceans (the pH scale is logarithmic, so a change of one in pH units is equivalent to a tenfold change in hydrogen ion concentration). Sea-surface pH and carbonate saturation states vary depending on ocean depth and location. Colder and higher latitude waters are capable of absorbing more . This can cause acidity to rise, lowering the pH and carbonate saturation levels in these areas. There are several other factors that influence the atmosphere-ocean exchange, and thus local ocean acidification. These include
ocean current
An ocean current is a continuous, directed movement of seawater generated by a number of forces acting upon the water, including wind, the Coriolis effect, breaking waves, cabbeling, and temperature and salinity differences. Depth contours, sh ...
s and
upwelling
Upwelling is an physical oceanography, oceanographic phenomenon that involves wind-driven motion of dense, cooler, and usually nutrient-rich water from deep water towards the ocean surface. It replaces the warmer and usually nutrient-depleted sur ...
zones, proximity to large continental rivers,
sea ice
Sea ice arises as seawater freezes. Because ice is less density, dense than water, it floats on the ocean's surface (as does fresh water ice). Sea ice covers about 7% of the Earth's surface and about 12% of the world's oceans. Much of the world' ...
coverage, and atmospheric exchange with
nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
and
sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
from
fossil fuel
A fossil fuel is a flammable carbon compound- or hydrocarbon-containing material formed naturally in the Earth's crust from the buried remains of prehistoric organisms (animals, plants or microplanktons), a process that occurs within geolog ...
burning and
agriculture
Agriculture encompasses crop and livestock production, aquaculture, and forestry for food and non-food products. Agriculture was a key factor in the rise of sedentary human civilization, whereby farming of domesticated species created ...
.
A lower ocean pH has a range of potentially harmful effects for
marine organisms
Marine life, sea life or ocean life is the collective ecological communities that encompass all aquatic animals, plants, algae, fungi, protists, single-celled microorganisms and associated viruses living in the saline water of marine habita ...
. Scientists have observed for example reduced calcification, lowered
immune response
An immune response is a physiological reaction which occurs within an organism in the context of inflammation for the purpose of defending against exogenous factors. These include a wide variety of different toxins, viruses, intra- and extracellula ...
s, and reduced energy for basic functions such as reproduction.
Ocean acidification can impact
marine ecosystem
Marine ecosystems are the largest of Earth's aquatic ecosystems and exist in Saline water, waters that have a high salt content. These systems contrast with freshwater ecosystems, which have a lower salt content. Marine waters cover more than 7 ...
s that provide food and livelihoods for many people. About one billion people are wholly or partially dependent on the fishing, tourism, and coastal management services provided by
coral reef
A coral reef is an underwater ecosystem characterized by reef-building corals. Reefs are formed of colonies of coral polyps held together by calcium carbonate. Most coral reefs are built from stony corals, whose polyps cluster in group ...
s. Ongoing acidification of the oceans may therefore threaten
food chain
A food chain is a linear network of links in a food web, often starting with an autotroph (such as grass or algae), also called a producer, and typically ending at an apex predator (such as grizzly bears or killer whales), detritivore (such as ...
s linked with the oceans.
One of the only solutions that would address the root cause of ocean acidification is reducing carbon dioxide emissions. This is one of the main objectives of
climate change mitigation
Climate change mitigation (or decarbonisation) is action to limit the greenhouse gases in the atmosphere that cause climate change. Climate change mitigation actions include energy conservation, conserving energy and Fossil fuel phase-out, repl ...
measures.
The removal of carbon dioxide from the atmosphere would also help to reverse ocean acidification. In addition, there are some specific
ocean-based mitigation methods, for example ocean
alkalinity
Alkalinity (from ) is the capacity of water to resist Freshwater acidification, acidification. It should not be confused with base (chemistry), basicity, which is an absolute measurement on the pH scale. Alkalinity is the strength of a buffer s ...
enhancement and
enhanced weathering
Enhanced weathering, also termed ocean alkalinity enhancement when proposed for carbon credit systems, is a process that aims to accelerate the natural weathering by spreading finely ground silicate rock, such as basalt, onto surfaces which speeds ...
. These strategies are under investigation, but generally have a low
technology readiness level
Technology readiness levels (TRLs) are a method for estimating the maturity of technologies during the acquisition phase of a program. TRLs enable consistent and uniform discussions of technical maturity across different types of technology. TR ...
and many risks.
Ocean acidification has happened before in Earth's geologic history.
The resulting ecological collapse in the oceans had long-lasting effects on the
global carbon cycle
The carbon cycle is a part of the biogeochemical cycle where carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of Earth. Other major biogeochemical cycles include the nitrogen cycle and the water cycl ...
and
climate
Climate is the long-term weather pattern in a region, typically averaged over 30 years. More rigorously, it is the mean and variability of meteorological variables over a time spanning from months to millions of years. Some of the meteoro ...
.
Cause

In 2021, atmospheric carbon dioxide (CO
2) levels of around 415 ppm were around 50% higher than preindustrial concentrations.
[ Text was copied from this source, which is available under ]
Creative Commons Attribution 4.0 International License
According to the National Oceanic and Atmospheric Administration in 2023, atmospheric CO2 levels have risen from approximately 280 parts per million (ppm) in the pre-industrial era to over 410 ppm today, primarily due to human activities such as fossil fuel combustion and deforestation. The current elevated levels and rapid growth rates are unprecedented in the past 55 million years of the geological record. The sources of this excess CO
2 are clearly established as human driven: they include anthropogenic fossil fuel, industrial, and land-use/land-change emissions. One source of this is fossil fuels, which are burned for energy. When burned, CO
2 is released into the atmosphere as a byproduct of combustion, which is a significant contributor to the increasing levels of CO
2 in the Earth's atmosphere. The ocean acts as a
carbon sink
A carbon sink is a natural or artificial carbon sequestration process that "removes a greenhouse gas, an aerosol or a precursor of a greenhouse gas from the atmosphere". These sinks form an important part of the natural carbon cycle. An overar ...
for anthropogenic CO
2 and takes up roughly a quarter of total anthropogenic CO
2 emissions.
[ Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License] However, the additional CO
2 in the ocean results in a wholesale shift in
seawater
Seawater, or sea water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approximat ...
acid-base chemistry toward more acidic, lower pH conditions and lower saturation states for carbonate minerals used in many marine organism shells and skeletons.
Accumulated since 1850, the ocean sink holds up to of carbon, with more than two-thirds of this amount ( C) being taken up by the global ocean since 1960. Over the historical period, the ocean sink increased in pace with the exponential anthropogenic emissions increase. From 1850 until 2022, the ocean has absorbed 26% of total anthropogenic emissions.
Emissions during the period 1850–2021 amounted to of carbon and were partitioned among the atmosphere (41%), ocean (26%), and land (31%).
The
carbon cycle
The carbon cycle is a part of the biogeochemical cycle where carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of Earth. Other major biogeochemical cycles include the nitrogen cycle and the water cycl ...
describes the fluxes of carbon dioxide () between the oceans,
terrestrial
Terrestrial refers to things related to land or the planet Earth, as opposed to extraterrestrial.
Terrestrial may also refer to:
* Terrestrial animal, an animal that lives on land opposed to living in water, or sometimes an animal that lives on o ...
biosphere
The biosphere (), also called the ecosphere (), is the worldwide sum of all ecosystems. It can also be termed the zone of life on the Earth. The biosphere (which is technically a spherical shell) is virtually a closed system with regard to mat ...
,
lithosphere
A lithosphere () is the rigid, outermost rocky shell of a terrestrial planet or natural satellite. On Earth, it is composed of the crust and the lithospheric mantle, the topmost portion of the upper mantle that behaves elastically on time ...
, and
atmosphere
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosph ...
. The carbon cycle involves both
organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
s such as
cellulose
Cellulose is an organic compound with the chemical formula, formula , a polysaccharide consisting of a linear chain of several hundred to many thousands of glycosidic bond, β(1→4) linked glucose, D-glucose units. Cellulose is an important s ...
and inorganic carbon compounds such as
carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
,
carbonate ion, and
bicarbonate ion
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial biochemical ...
, together referenced as dissolved inorganic carbon (DIC). These inorganic compounds are particularly significant in ocean acidification, as they include many forms of dissolved present in the Earth's oceans.
When dissolves, it reacts with water to form a balance of ionic and non-ionic chemical species: dissolved free carbon dioxide (),
carbonic acid
Carbonic acid is a chemical compound with the chemical formula . The molecule rapidly converts to water and carbon dioxide in the presence of water. However, in the absence of water, it is quite stable at room temperature. The interconversion ...
(),
bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial bioche ...
() and carbonate (). The ratio of these species depends on factors such as
seawater
Seawater, or sea water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approximat ...
temperature
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making ...
, pressure and salinity (as shown in a
Bjerrum plot). These different forms of
dissolved inorganic carbon
Dissolved inorganic carbon (DIC) is the sum of the aqueous species of inorganic carbon in a solution. Carbon compounds can be distinguished as either organic or inorganic, and as dissolved or particulate, depending on their composition. Organic c ...
are transferred from an ocean's surface to its interior by the ocean's
solubility pump
In oceanic biogeochemistry, the solubility pump is a physico-chemical process that transports carbon as dissolved inorganic carbon (DIC) from the ocean's surface to its interior.
Overview
The solubility pump is driven by the coincidence of two ...
. The resistance of an area of ocean to absorbing atmospheric is known as the
Revelle factor.
Main effects
The
ocean's chemistry is changing due to the uptake of anthropogenic carbon dioxide (CO
2).
[ Text was copied from this source, which is available under ]
Creative Commons Attribution 4.0 International License
[Cooley, S., D. Schoeman, L. Bopp, P. Boyd, S. Donner, D.Y. Ghebrehiwet, S.-I. Ito, W. Kiessling, P. Martinetto, E. Ojea, M.-F. Racault, B. Rost, and M. Skern-Mauritzen, 2022]
Chapter 3: Oceans and Coastal Ecosystems and Their Services
. In
Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change
.-O. Pörtner, D.C. Roberts, M. Tignor, E.S. Poloczanska, K. Mintenbeck, A. Alegría, M. Craig, S. Langsdorf, S. Löschke, V. Möller, A. Okem, B. Rama (eds.) Cambridge University Press, Cambridge, UK and New York, NY, US, pp. 379–550. Ocean pH, carbonate ion concentrations (
O32−, and calcium carbonate mineral saturation states (Ω) have been declining as a result of the uptake of approximately 30% of the anthropogenic carbon dioxide emissions over the past 270 years (since around 1750). This process, commonly referred to as "ocean acidification", is making it harder for
marine calcifiers to build a shell or skeletal structure, endangering coral reefs and the broader marine ecosystems.
Ocean acidification has been called the "evil twin of
global warming
Present-day climate change includes both global warming—the ongoing increase in global average temperature—and its wider effects on Earth's climate system. Climate change in a broader sense also includes previous long-term changes ...
" and "the other CO
2 problem".
Increased ocean temperatures and oxygen loss act concurrently with ocean acidification and constitute the "deadly trio" of climate change pressures on the marine environment. The impacts of this will be most severe for coral reefs and other shelled marine organisms, as well as those populations that depend on the ecosystem services they provide.
Reduction in pH value
Dissolving in seawater increases the
hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
ion () concentration in the ocean, and thus decreases ocean pH, as follows:
In shallow coastal and shelf regions, a number of factors interplay to affect air-ocean exchange and resulting pH change.
These include biological processes, such as
photosynthesis
Photosynthesis ( ) is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabo ...
and respiration, as well as water upwelling. Also,
ecosystem metabolism in freshwater sources reaching coastal waters can lead to large, but local, pH changes.
Freshwater bodies also appear to be acidifying, although this is a more complex and less obvious phenomenon.
The absorption of CO
2 from the atmosphere does not affect the ocean's
alkalinity
Alkalinity (from ) is the capacity of water to resist Freshwater acidification, acidification. It should not be confused with base (chemistry), basicity, which is an absolute measurement on the pH scale. Alkalinity is the strength of a buffer s ...
.
This is important to know in this context as alkalinity is the capacity of
water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
to resist
acidification
Acidification may refer to:
* Ocean acidification, decrease in the pH of the Earth's oceans
* Freshwater acidification, atmospheric depositions and soil leaching of SOx and NOx
* Soil acidification, buildup of hydrogen cations, which reduces the ...
.
Ocean alkalinity enhancement
Ocean acidification is the ongoing decrease in the pH of the Earth's ocean. Between 1950 and 2020, the average pH of the ocean surface fell from approximately 8.15 to 8.05. Carbon dioxide emissions from human activities are the primary cause of ...
has been proposed as one option to add alkalinity to the ocean and therefore buffer against pH changes.
Decreased calcification in marine organisms
Changes in ocean chemistry can have extensive direct and indirect effects on organisms and their habitats. One of the most important repercussions of increasing ocean acidity relates to the production of shells out of
calcium carbonate
Calcium carbonate is a chemical compound with the chemical formula . It is a common substance found in Rock (geology), rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skel ...
().
This process is called calcification and is important to the biology and survival of a wide range of marine organisms. Calcification involves the
precipitation
In meteorology, precipitation is any product of the condensation of atmospheric water vapor that falls from clouds due to gravitational pull. The main forms of precipitation include drizzle, rain, rain and snow mixed ("sleet" in Commonwe ...
of dissolved ions into solid structures, structures for many marine organisms, such as
coccolithophore
Coccolithophores, or coccolithophorids, are single-celled organisms which are part of the phytoplankton, the autotrophic (self-feeding) component of the plankton community. They form a group of about 200 species, and belong either to the kingdom ...
s,
foraminifera
Foraminifera ( ; Latin for "hole bearers"; informally called "forams") are unicellular organism, single-celled organisms, members of a phylum or class (biology), class of Rhizarian protists characterized by streaming granular Ectoplasm (cell bio ...
,
crustacea
Crustaceans (from Latin meaning: "those with shells" or "crusted ones") are invertebrate animals that constitute one group of arthropods that are traditionally a part of the subphylum Crustacea (), a large, diverse group of mainly aquatic arthrop ...
ns,
mollusks
Mollusca is a phylum of protostomic invertebrate animals, whose members are known as molluscs or mollusks (). Around 76,000 extant species of molluscs are recognized, making it the second-largest animal phylum after Arthropoda. The num ...
, etc. After they are formed, these structures are vulnerable to
dissolution unless the surrounding seawater contains
saturating concentrations of carbonate ions ().
Very little of the extra carbon dioxide that is added into the ocean remains as dissolved carbon dioxide. The majority dissociates into additional bicarbonate and free hydrogen ions. The increase in hydrogen is larger than the increase in bicarbonate,
creating an imbalance in the reaction:
:
To maintain chemical equilibrium, some of the carbonate ions already in the ocean combine with some of the hydrogen ions to make further bicarbonate. Thus the ocean's concentration of carbonate ions is reduced, removing an essential building block for marine organisms to build shells, or calcify:
:
The increase in concentrations of dissolved carbon dioxide and bicarbonate, and reduction in carbonate, are shown in the
Bjerrum plot.
Disruption of the food chain is also a possible effect as many marine organisms rely on calcium carbonate-based organisms at the base of the food chain for food and habitat. This can potentially have detrimental effects throughout the food web and potentially lead to a decline in availability of fish stocks which would have an impact on human livelihoods.
Decrease in saturation state
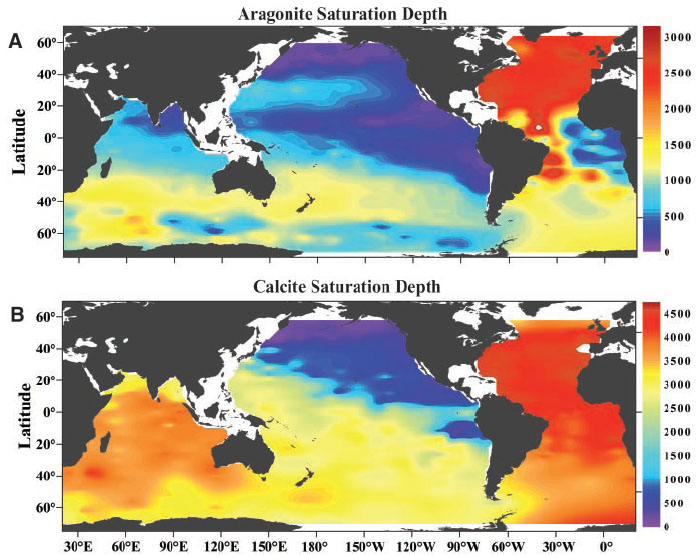
The
saturation
Saturation, saturated, unsaturation or unsaturated may refer to:
Chemistry
*Saturated and unsaturated compounds, a classification of compounds related to their ability to resist addition reactions
** Degree of unsaturation
**Saturated fat or satu ...
state (known as Ω) of seawater for a mineral is a measure of the thermodynamic potential for the mineral to form or to dissolve, and for calcium carbonate is described by the following equation:
:
Here Ω is the product of the concentrations (or
activities) of the reacting ions that form the mineral (Ca
2+ and CO
32−), divided by the apparent solubility product at
equilibrium
Equilibrium may refer to:
Film and television
* ''Equilibrium'' (film), a 2002 science fiction film
* '' The Story of Three Loves'', also known as ''Equilibrium'', a 1953 romantic anthology film
* "Equilibrium" (''seaQuest 2032'')
* ''Equilibr ...
(K
sp), that is, when the rates of precipitation and dissolution are equal. In seawater, dissolution boundary is formed as a result of temperature, pressure, and depth, and is known as the saturation horizon.
Above this saturation horizon, Ω has a value greater than 1, and does not readily dissolve. Most calcifying organisms live in such waters.
Below this depth, Ω has a value less than 1, and will dissolve. The
carbonate compensation depth
The carbonate compensation depth (CCD) is the depth, in the oceans, at which the rate of supply of calcium carbonates matches the rate of solvation. That is, solvation 'compensates' supply. Below the CCD solvation is faster, so that carbonate pa ...
is the ocean depth at which carbonate dissolution balances the supply of carbonate to sea floor, therefore sediment below this depth will be void of calcium carbonate. Increasing levels, and the resulting lower pH of seawater, decreases the concentration of CO
32− and the saturation state of therefore increasing dissolution.
Calcium carbonate most commonly occurs in two common
polymorphs (crystalline forms):
aragonite
Aragonite is a carbonate mineral and one of the three most common naturally occurring crystal forms of calcium carbonate (), the others being calcite and vaterite. It is formed by biological and physical processes, including precipitation fr ...
and
calcite
Calcite is a Carbonate minerals, carbonate mineral and the most stable Polymorphism (materials science), polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on ...
. Aragonite is much more soluble than calcite, so the aragonite saturation horizon, and aragonite compensation depth, is always nearer to the surface than the calcite saturation horizon.
This also means that those organisms that produce aragonite may be more vulnerable to changes in ocean acidity than those that produce calcite.
Ocean acidification and the resulting decrease in carbonate saturation states raise the saturation horizons of both forms closer to the surface.
This decrease in saturation state is one of the main factors leading to decreased calcification in marine organisms because the inorganic precipitation of is directly proportional to its saturation state and calcifying organisms exhibit stress in waters with lower saturation states.
Natural variability and climate feedbacks
Already now large quantities of water undersaturated in
aragonite
Aragonite is a carbonate mineral and one of the three most common naturally occurring crystal forms of calcium carbonate (), the others being calcite and vaterite. It is formed by biological and physical processes, including precipitation fr ...
are
upwelling
Upwelling is an physical oceanography, oceanographic phenomenon that involves wind-driven motion of dense, cooler, and usually nutrient-rich water from deep water towards the ocean surface. It replaces the warmer and usually nutrient-depleted sur ...
close to the Pacific
continental shelf
A continental shelf is a portion of a continent that is submerged under an area of relatively shallow water, known as a shelf sea. Much of these shelves were exposed by drops in sea level during glacial periods. The shelf surrounding an islan ...
area of North America, from
Vancouver
Vancouver is a major city in Western Canada, located in the Lower Mainland region of British Columbia. As the List of cities in British Columbia, most populous city in the province, the 2021 Canadian census recorded 662,248 people in the cit ...
to
Northern California
Northern California (commonly shortened to NorCal) is a geocultural region that comprises the northern portion of the U.S. state of California, spanning the northernmost 48 of the state's List of counties in California, 58 counties. Northern Ca ...
.
These continental shelves play an important role in marine ecosystems, since most
marine organisms
Marine life, sea life or ocean life is the collective ecological communities that encompass all aquatic animals, plants, algae, fungi, protists, single-celled microorganisms and associated viruses living in the saline water of marine habita ...
live or are
spawned there. Other shelf areas may be experiencing similar effects.
At depths of 1000s of meters in the ocean, calcium carbonate shells begin to dissolve as increasing pressure and decreasing temperature shift the chemical equilibria controlling calcium carbonate precipitation.
The depth at which this occurs is known as the
carbonate compensation depth
The carbonate compensation depth (CCD) is the depth, in the oceans, at which the rate of supply of calcium carbonates matches the rate of solvation. That is, solvation 'compensates' supply. Below the CCD solvation is faster, so that carbonate pa ...
. Ocean acidification will increase such dissolution and shallow the carbonate compensation depth on timescales of tens to hundreds of years.
Zones of
downwelling
Downwelling is the downward movement of a fluid parcel and its properties (e.g., salinity, temperature, pH) within a larger fluid. It is closely related to upwelling, the upward movement of fluid.
While downwelling is most commonly used to des ...
are being affected first.
In the North Pacific and North Atlantic, saturation states are also decreasing (the depth of saturation is getting more shallow).
Ocean acidification is progressing in the open ocean as the CO
2 travels to deeper depth as a result of ocean mixing. In the open ocean, this causes carbonate compensation depths to become more shallow, meaning that dissolution of calcium carbonate will occur below those depths. In the North Pacific these carbonate saturations depths are shallowing at a rate of .
It is expected that ocean acidification in the future will lead to a significant decrease in the burial of carbonate sediments for several centuries, and even the dissolution of existing carbonate sediments.
Measured and estimated values
Present day and recent history

Between 1950 and 2020, the average pH value of the ocean surface is estimated to have decreased from approximately 8.15 to 8.05.
[ This represents an increase of around 26% in hydrogen ion concentration in the world's oceans (the pH scale is logarithmic, so a change of one in pH unit is equivalent to a tenfold change in hydrogen ion concentration).]IPCC Sixth Assessment Report
The Sixth Assessment Report (AR6) of the United Nations (UN) Intergovernmental Panel on Climate Change (IPCC) is the sixth in a series of reports which assess the available scientific information on climate change. Three Working Groups (WGI, II, ...
in 2021 stated that "present-day surface pH values are unprecedented for at least 26,000 years and current rates of pH change are unprecedented since at least that time.[Arias, P.A., N. Bellouin, E. Coppola, R.G. Jones, G. Krinner, J. Marotzke, V. Naik, M.D. Palmer, G.-K. Plattner, J. Rogelj, M. Rojas, J. Sillmann, T. Storelvmo, P.W. Thorne, B. Trewin, K. Achuta Rao, B. Adhikary, R.P. Allan, K. Armour, G. Bala, R. Barimalala, S. Berger, J.G. Canadell, C. Cassou, A. Cherchi, W. Collins, W.D. Collins, S.L. Connors, S. Corti, F. Cruz, F.J. Dentener, C. Dereczynski, A. Di Luca, A. Diongue Niang, F.J. Doblas-Reyes, A. Dosio, H. Douville, F. Engelbrecht, V. Eyring, E. Fischer, P. Forster, B. Fox-Kemper, J.S. Fuglestvedt, J.C. Fyfe, et al., 2021]
Technical Summary
. I
Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change
[Masson-Delmotte, V., P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, US, pp. 33–144. The report also found that "pH in open ocean surface water has declined by about 0.017 to 0.027 pH units per decade since the late 1980s".[Canadell, J.G., P.M.S. Monteiro, M.H. Costa, L. Cotrim da Cunha, P.M. Cox, A.V. Eliseev, S. Henson, M. Ishii, S. Jaccard, C. Koven, A. Lohila, P.K. Patra, S. Piao, J. Rogelj, S. Syampungani, S. Zaehle, and K. Zickfeld, 2021]
Chapter 5: Global Carbon and other Biogeochemical Cycles and Feedbacks
. I
Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change
[Masson-Delmotte, V., P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, US, pp. 673–816.
The rate of decline differs by region. This is due to complex interactions between different types of forcing mechanisms:upwelling
Upwelling is an physical oceanography, oceanographic phenomenon that involves wind-driven motion of dense, cooler, and usually nutrient-rich water from deep water towards the ocean surface. It replaces the warmer and usually nutrient-depleted sur ...
of -rich sub-surface waters in addition to anthropogenic uptake."
Geologic past
Ocean acidification has occurred previously in Earth's history.Capitanian mass extinction
In the geologic timescale, the Capitanian is an age (geology), age or stage (stratigraphy), stage of the Permian. It is also the uppermost or latest of three subdivisions of the Guadalupian Epoch (geology), Epoch or series (stratigraphy), Series. ...
,[Bond, D.P.G., Wignall, P.B., Joachimski, M.M., Sun, Y., Savov, I., Grasby, S.E., Beauchamp, B. and Blomeier, D.P. 2015]
An abrupt extinction in the Middle Permian (Capitanian) of the Boreal Realm (Spitsbergen) and its link to anoxia and acidification
''Geological Society of America Bulletin'', 127 (9–10): 1411–1421.volcanism
Volcanism, vulcanism, volcanicity, or volcanic activity is the phenomenon where solids, liquids, gases, and their mixtures erupt to the surface of a solid-surface astronomical body such as a planet or a moon. It is caused by the presence of a he ...
and/or thermal dissociation of marine gas hydrates
Clathrate hydrates, or gas hydrates, clathrates, or hydrates, are crystalline water-based solids physically resembling ice, in which small non-polar molecules (typically gases) or polar molecules with large hydrophobic moieties are trapped ins ...
.Triassic
The Triassic ( ; sometimes symbolized 🝈) is a geologic period and system which spans 50.5 million years from the end of the Permian Period 251.902 million years ago ( Mya), to the beginning of the Jurassic Period 201.4 Mya. The Triassic is t ...
.
Predicted future values
 Importantly, the rate of change in ocean acidification is much higher than in the geological past. This faster change prevents organisms from gradually adapting, and prevents climate cycle feedbacks from kicking in to mitigate ocean acidification. Ocean acidification is now on a path to reach lower pH levels than at any other point in the last 300 million years.
Importantly, the rate of change in ocean acidification is much higher than in the geological past. This faster change prevents organisms from gradually adapting, and prevents climate cycle feedbacks from kicking in to mitigate ocean acidification. Ocean acidification is now on a path to reach lower pH levels than at any other point in the last 300 million years.biogeochemical
Biogeochemistry is the scientific discipline that involves the study of the chemical, physical, geological, and biological processes and reactions that govern the composition of the natural environment (including the biosphere, the cryosphere, ...
changes, this drop in pH value could undermine the functioning of marine ecosystems and disrupt the provision of many goods and services associated with the ocean, beginning as early as 2100.climate change mitigation
Climate change mitigation (or decarbonisation) is action to limit the greenhouse gases in the atmosphere that cause climate change. Climate change mitigation actions include energy conservation, conserving energy and Fossil fuel phase-out, repl ...
efforts taken by nations and their governments.socioeconomic
Economics () is a behavioral science that studies the Production (economics), production, distribution (economics), distribution, and Consumption (economics), consumption of goods and services.
Economics focuses on the behaviour and interac ...
global changes are modelled by using the Shared Socioeconomic Pathways
Shared Socioeconomic Pathways (SSPs) are climate change scenarios of projected socioeconomic global changes up to 2100 as defined in the IPCC Sixth Assessment Report on climate change in 2021. They are used to derive greenhouse gas emissions sc ...
(SSP) scenarios.
Under a very high emission scenario (SSP5-8.5), model projections estimate that surface ocean pH could decrease by as much as 0.44 units by the end of this century, compared to the end of the 19th century. This would mean a pH as low as about 7.7, and represents a further increase in H+ concentrations of two to four times beyond the increase to date.
Impacts on oceanic calcifying organisms

Complexity of research findings
The full ecological consequences of the changes in calcification due to ocean acidification are complex but it appears likely that many calcifying species will be adversely affected by ocean acidification.food chain
A food chain is a linear network of links in a food web, often starting with an autotroph (such as grass or algae), also called a producer, and typically ending at an apex predator (such as grizzly bears or killer whales), detritivore (such as ...
from autotroph
An autotroph is an organism that can convert Abiotic component, abiotic sources of energy into energy stored in organic compounds, which can be used by Heterotroph, other organisms. Autotrophs produce complex organic compounds (such as carbohy ...
s to heterotroph
A heterotroph (; ) is an organism that cannot produce its own food, instead taking nutrition from other sources of organic carbon, mainly plant or animal matter. In the food chain, heterotrophs are primary, secondary and tertiary consumers, but ...
s and include organisms such as coccolithophore
Coccolithophores, or coccolithophorids, are single-celled organisms which are part of the phytoplankton, the autotrophic (self-feeding) component of the plankton community. They form a group of about 200 species, and belong either to the kingdom ...
s, coral
Corals are colonial marine invertebrates within the subphylum Anthozoa of the phylum Cnidaria. They typically form compact Colony (biology), colonies of many identical individual polyp (zoology), polyps. Coral species include the important Coral ...
s, foraminifera
Foraminifera ( ; Latin for "hole bearers"; informally called "forams") are unicellular organism, single-celled organisms, members of a phylum or class (biology), class of Rhizarian protists characterized by streaming granular Ectoplasm (cell bio ...
, echinoderm
An echinoderm () is any animal of the phylum Echinodermata (), which includes starfish, brittle stars, sea urchins, sand dollars and sea cucumbers, as well as the sessile sea lilies or "stone lilies". While bilaterally symmetrical as ...
s, crustacea
Crustaceans (from Latin meaning: "those with shells" or "crusted ones") are invertebrate animals that constitute one group of arthropods that are traditionally a part of the subphylum Crustacea (), a large, diverse group of mainly aquatic arthrop ...
ns and molluscs
Mollusca is a phylum of protostome, protostomic invertebrate animals, whose members are known as molluscs or mollusks (). Around 76,000 extant taxon, extant species of molluscs are recognized, making it the second-largest animal phylum ...
.[National Research Council. ]
Overview of Climate Changes and Illustrative Impacts. Climate Stabilization Targets: Emissions, Concentrations, and Impacts over Decades to Millennia
.'' Washington, DC: The National Academies Press, 2011. 1. Print.
Overall, all marine ecosystems on Earth will be exposed to changes in acidification and several other ocean biogeochemical changes. Ocean acidification may force some organisms to reallocate resources away from productive endpoints in order to maintain calcification. For example, the oyster ''Magallana gigas
The Pacific oyster, Japanese oyster, or Miyagi oyster (''Magallana gigas'') is an oyster native to the Pacific coast of Asia. It has become an introduced species in North America, Australia, Europe, and New Zealand.
Etymology
The genus ''Magal ...
'' is recognized to experience metabolic changes alongside altered calcification
Calcification is the accumulation of calcium salts in a body tissue. It normally occurs in the formation of bone, but calcium can be deposited abnormally in soft tissue,Miller, J. D. Cardiovascular calcification: Orbicular origins. ''Nature M ...
rates due to energetic tradeoffs resulting from pH imbalances.supersaturated
In physical chemistry, supersaturation occurs with a solution when the concentration of a solute exceeds the concentration specified by the value of solubility at equilibrium. Most commonly the term is applied to a solution of a solid in a ...
with respect to seawater. However, as ocean pH falls, the concentration of carbonate ions also decreases. Calcium carbonate thus becomes undersaturated, and structures made of calcium carbonate are vulnerable to calcification stress and dissolution. In particular, studies show that corals,pteropods
Pteropoda (common name pteropods, from the Greek meaning "wing-foot") are specialized free-swimming pelagic sea snails and sea slugs, marine opisthobranch gastropods. Most live in the top 10 m of the ocean and are less than 1 cm long. ...
marine conservation
Marine conservation, also known as ocean conservation, is the protection and preservation of ecosystems in oceans and seas through planned management in order to prevent the over-exploitation of these marine resources. Marine conservation is i ...
practices it may be impossible to bring back many previous shellfish populations.primary production
In ecology, primary production is the synthesis of organic compounds from atmospheric or aqueous carbon dioxide. It principally occurs through the process of photosynthesis, which uses light as its source of energy, but it also occurs through ...
and calcification in response to elevated ,Pisaster ochraceus
''Pisaster ochraceus'', generally known as the purple sea star, ochre sea star, or ochre starfish, is a common seastar found among the waters of the Pacific Ocean. Identified as a keystone species, ''P. ochraceus'' is considered an important ind ...
, shows enhanced growth in waters with increased acidity.carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
from the atmosphere to the ocean interior and seafloor sediment
Marine sediment, or ocean sediment, or seafloor sediment, are deposits of insoluble particles that have accumulated on the seafloor. These particles either have their origins in soil and rocks and have been transported from the land to the ...
, weakening the so-called biological pump
The biological pump (or ocean carbon biological pump or marine biological carbon pump) is the ocean's biologically driven Carbon sequestration, sequestration of carbon from the atmosphere and land runoff to the ocean interior and seafloor sedim ...
.
Coccolithophores
A coccolithophore is a unicellular
A unicellular organism, also known as a single-celled organism, is an organism that consists of a single cell, unlike a multicellular organism that consists of multiple cells. Organisms fall into two general categories: prokaryotic organisms and ...
, eukaryotic
The eukaryotes ( ) constitute the Domain (biology), domain of Eukaryota or Eukarya, organisms whose Cell (biology), cells have a membrane-bound cell nucleus, nucleus. All animals, plants, Fungus, fungi, seaweeds, and many unicellular organisms ...
phytoplankton
Phytoplankton () are the autotrophic (self-feeding) components of the plankton community and a key part of ocean and freshwater Aquatic ecosystem, ecosystems. The name comes from the Greek language, Greek words (), meaning 'plant', and (), mea ...
(alga
Algae ( , ; : alga ) is an informal term for any organisms of a large and diverse group of photosynthetic organisms that are not plants, and includes species from multiple distinct clades. Such organisms range from unicellular microalgae, suc ...
). Understanding calcification changes in coccolithophores may be particularly important because a decline in the coccolithophores may have secondary effects on climate: it could contribute to global warming by decreasing the Earth's albedo
Albedo ( ; ) is the fraction of sunlight that is Diffuse reflection, diffusely reflected by a body. It is measured on a scale from 0 (corresponding to a black body that absorbs all incident radiation) to 1 (corresponding to a body that reflects ...
via their effects on oceanic cloud cover.North Atlantic
The Atlantic Ocean is the second largest of the world's five oceanic divisions, with an area of about . It covers approximately 17% of Earth's surface and about 24% of its water surface area. During the Age of Discovery, it was known for ...
and found that the species composition of coccolithophorids remained unchanged over the past 224 years (1780 to 2004). But the average coccolith mass had increased by 40% during the same period.
Corals
Warm water corals are clearly in decline, with losses of 50% over the last 30–50 years due to multiple threats from ocean warming, ocean acidification, pollution
Pollution is the introduction of contaminants into the natural environment that cause harm. Pollution can take the form of any substance (solid, liquid, or gas) or energy (such as radioactivity, heat, sound, or light). Pollutants, the component ...
and physical damage from activities such as fishing, and these pressures are expected to intensify.exoskeleton
An exoskeleton () . is a skeleton that is on the exterior of an animal in the form of hardened integument, which both supports the body's shape and protects the internal organs, in contrast to an internal endoskeleton (e.g. human skeleton, that ...
is also extremely important for calcification growth. When the saturation state of aragonite in the external seawater is at ambient levels, the corals will grow their aragonite crystals rapidly in their internal compartments, hence their exoskeleton grows rapidly. If the saturation state of aragonite in the external seawater is lower than the ambient level, the corals have to work harder to maintain the right balance in the internal compartment. When that happens, the process of growing the crystals slows down, and this slows down the rate of how much their exoskeleton is growing. Depending on the aragonite saturation state in the surrounding water, the corals may halt growth because pumping aragonite into the internal compartment will not be energetically favorable. Under the current progression of carbon emissions, around 70% of North Atlantic cold-water corals will be living in corrosive waters by 2050–60.Great Barrier Reef
The Great Barrier Reef is the world's largest coral reef system, composed of over 2,900 individual reefs and 900 islands stretching for over over an area of approximately . The reef is located in the Coral Sea, off the coast of Queensland, ...
, to decrease seawater CO2 level (raise pH) to near the preindustrial value showed a 7% increase in net calcification.Queensland
Queensland ( , commonly abbreviated as Qld) is a States and territories of Australia, state in northeastern Australia, and is the second-largest and third-most populous state in Australia. It is bordered by the Northern Territory, South Austr ...
and Western Australia
Western Australia (WA) is the westernmost state of Australia. It is bounded by the Indian Ocean to the north and west, the Southern Ocean to the south, the Northern Territory to the north-east, and South Australia to the south-east. Western Aust ...
from 2007 to 2012 found that corals are more resistant to the environmental pH changes than previously thought, due to internal homeostasis
In biology, homeostasis (British English, British also homoeostasis; ) is the state of steady internal physics, physical and chemistry, chemical conditions maintained by organism, living systems. This is the condition of optimal functioning fo ...
regulation; this makes thermal change (marine heatwave
A marine heatwave is a period of abnormally high sea surface temperatures compared to the typical temperatures in the past for a particular season and region. Marine heatwaves are caused by a variety of drivers. These include shorter term weather ...
s), which leads to coral bleaching
Coral bleaching is the process when corals become white due to loss of Symbiosis, symbiotic algae and Photosynthesis, photosynthetic pigments. This loss of pigment can be caused by various stressors, such as changes in water temperature, light, ...
, rather than acidification, the main factor for coral reef vulnerability due to climate change.
Studies at carbon dioxide seep sites
In some places carbon dioxide bubbles out from the sea floor, locally changing the pH and other aspects of the chemistry of the seawater. Studies of these carbon dioxide seeps have documented a variety of responses by different organisms.Papua New Guinea
Papua New Guinea, officially the Independent State of Papua New Guinea, is an island country in Oceania that comprises the eastern half of the island of New Guinea and offshore islands in Melanesia, a region of the southwestern Pacific Ocean n ...
, declining pH caused by carbon dioxide seeps is associated with declines in coral species diversity. However, in Palau
Palau, officially the Republic of Palau, is an island country in the Micronesia subregion of Oceania in the western Pacific Ocean. The Republic of Palau consists of approximately 340 islands and is the western part of the Caroline Islands ...
carbon dioxide seeps are not associated with reduced species diversity of corals, although bioerosion
Bioerosion describes the breakdown of hard ocean substrates – and less often terrestrial substrates – by living organisms. Marine bioerosion can be caused by mollusks, polychaete worms, phoronids, sponges, crustaceans, echinoids, ...
of coral skeletons is much higher at low pH sites.
Pteropods and brittle stars
Pteropods
Pteropoda (common name pteropods, from the Greek meaning "wing-foot") are specialized free-swimming pelagic sea snails and sea slugs, marine opisthobranch gastropods. Most live in the top 10 m of the ocean and are less than 1 cm long. ...
and brittle star
Brittle stars, serpent stars, or ophiuroids (; ; referring to the serpent-like arms of the brittle star) are echinoderms in the class Ophiuroidea, closely related to starfish. They crawl across the sea floor using their flexible arms for locomot ...
s both form the base of the Arctic food web
A food web is the natural interconnection of food chains and a graphical representation of what-eats-what in an ecological community. Position in the food web, or trophic level, is used in ecology to broadly classify organisms as autotrophs or he ...
s and are both seriously damaged from acidification. Pteropods shells dissolve with increasing acidification and the brittle stars lose muscle mass when re-growing appendage
An appendage (or outgrowth) is an external body part or natural prolongation that protrudes from an organism's body such as an arm or a leg. Protrusions from single-celled bacteria and archaea are known as cell-surface appendages or surface app ...
s. For pteropods to create shells they require aragonite which is produced through carbonate ions and dissolved calcium and strontium. Pteropods are severely affected because increasing acidification levels have steadily decreased the amount of water supersaturated with carbonate.brittle star
Brittle stars, serpent stars, or ophiuroids (; ; referring to the serpent-like arms of the brittle star) are echinoderms in the class Ophiuroidea, closely related to starfish. They crawl across the sea floor using their flexible arms for locomot ...
, a relative of the common sea star
Starfish or sea stars are Star polygon, star-shaped echinoderms belonging to the class (biology), class Asteroidea (). Common usage frequently finds these names being also applied to brittle star, ophiuroids, which are correctly referred to ...
, fewer than 0.1 percent survived more than eight days.
Other impacts on ecosystems
Other biological impacts
Aside from the slowing and/or reversal of calcification, organisms may suffer other adverse effects, either indirectly through negative impacts on food resources, or directly as reproductive or physiological effects.hypercapnia
Hypercapnia (from the Greek ''hyper'', "above" or "too much" and ''kapnos'', "smoke"), also known as hypercarbia and CO2 retention, is a condition of abnormally elevated carbon dioxide (CO2) levels in the blood. Carbon dioxide is a gaseous pro ...
.
Increasing acidity has been observed to reduce metabolic rates in jumbo squid
The Humboldt squid (''Dosidicus gigas''), also known as jumbo squid or jumbo flying squid, is a large, predatory squid living in the eastern Pacific Ocean. It is the only known species of the genus ''Dosidicus'' of the subfamily Ommastrephinae, ...
Atlantic longfin squid
The Atlantic Ocean is the second largest of the world's five oceanic divisions, with an area of about . It covers approximately 17% of Earth's surface and about 24% of its water surface area. During the Age of Discovery, it was known for se ...
eggs took longer to hatch in acidified water, and the squid's statolith was smaller and malformed in animals placed in sea water with a lower pH. However, these studies are ongoing and there is not yet a full understanding of these processes in marine organisms or ecosystem
An ecosystem (or ecological system) is a system formed by Organism, organisms in interaction with their Biophysical environment, environment. The Biotic material, biotic and abiotic components are linked together through nutrient cycles and en ...
s.
Acoustic properties
Another potential route to ecosystem impacts is through bioacoustics
Bioacoustics is a cross-disciplinary science that combines biology and acoustics. Usually it refers to the investigation of sound production, dispersion and reception in animals (including humans). This involves neurophysiology, neurophysiological ...
. This may occur as ocean acidification can alter the acoustic properties of seawater, allowing sound to propagate further, and increasing ocean noise.communication
Communication is commonly defined as the transmission of information. Its precise definition is disputed and there are disagreements about whether Intention, unintentional or failed transmissions are included and whether communication not onl ...
.[Acid In The Oceans: A Growing Threat To Sea Life](_blank)
by Richard Harris. All Things Considered, 12 August 2009.
Algae and seagrasses
Another possible effect would be an increase in harmful algal bloom
A harmful algal bloom (HAB), or excessive algae growth, sometimes called a red tide in marine environments, is an algal bloom that causes negative impacts to other organisms by production of natural algae-produced toxins, water deoxygenation, ...
events, which could contribute to the accumulation of toxins (domoic acid
Domoic acid (DA) is a kainic acid-type neurotoxin that causes amnesic shellfish poisoning (ASP). It is produced by algae and accumulates in shellfish, sardines, and anchovies. When sea lions, otters, cetaceans, humans, and other predators eat cont ...
, brevetoxin
Brevetoxin (PbTx), or brevetoxins, are a suite of cyclic polyether compounds produced naturally by a species of dinoflagellate known as ''Karenia brevis''. Brevetoxins are neurotoxins that bind to voltage-gated sodium channels in nerve cells, lea ...
, saxitoxin
Saxitoxin (STX) is a potent neurotoxin and the best-known paralytic shellfish toxin. Ingestion of saxitoxin by humans, usually by consumption of shellfish contaminated by toxic algal blooms, is responsible for the illness known as paralytic she ...
) in small organisms such as anchovies
An anchovy is a small, common forage fish of the family Engraulidae. Most species are found in marine waters, but several will enter brackish water, and some in South America are restricted to fresh water.
More than 140 species are placed in 1 ...
and shellfish
Shellfish, in colloquial and fisheries usage, are exoskeleton-bearing Aquatic animal, aquatic invertebrates used as Human food, food, including various species of Mollusca, molluscs, crustaceans, and echinoderms. Although most kinds of shellfish ...
, in turn increasing occurrences of amnesic shellfish poisoning
Amnesic shellfish poisoning (ASP) is an illness caused by consumption of shellfish that contain the marine biotoxin called domoic acid. In mammals, including humans, domoic acid acts as a neurotoxin
Neurotoxins are toxins that are destructive ...
, neurotoxic shellfish poisoning
Neurotoxic shellfish poisoning (NSP) is caused by the consumption of brevetoxins, which are marine toxins produced by the dinoflagellate ''Karenia brevis'' (among several others). These toxins can produce a series of gastrointestinal and neurolo ...
and paralytic shellfish poisoning
Paralytic shellfish poisoning (PSP) is one of the four recognized syndromes of shellfish poisoning, which share some common features and are primarily associated with bivalve mollusks (such as mussels, clams, oysters and scallops). These shellfi ...
.
Fish larvae
Ocean acidification can also have effects on marine fish larvae
Ichthyoplankton (from Greek: wikt:ἰχθύς, ἰχθύς, , "fish"; and πλαγκτός, , "drifter") are the Fish eggs, eggs and larvae of fish. They are mostly found in the sunlit zone of the water column, less than 200 metres deep, which ...
. It internally affects their olfactory systems, which is a crucial part of their early development. Orange clownfish
The orange clownfish (''Amphiprion percula'') also known as percula clownfish and clown anemonefish, is widely known as a popular aquarium fish. Like other clownfishes (also known as anemonefishes), it often lives in association with sea anemones. ...
larvae mostly live on oceanic reefs that are surrounded by vegetative islands.meta-analysis
Meta-analysis is a method of synthesis of quantitative data from multiple independent studies addressing a common research question. An important part of this method involves computing a combined effect size across all of the studies. As such, th ...
published in 2022 found that the effect size
In statistics, an effect size is a value measuring the strength of the relationship between two variables in a population, or a sample-based estimate of that quantity. It can refer to the value of a statistic calculated from a sample of data, the ...
s of published studies testing for ocean acidification effects on fish behavior have declined by an order of magnitude over the past decade, and have been negligible for the past five years.European eel
The European eel (''Anguilla anguilla'') is a species of eel. Their life history was a mystery for thousands of years, and mating in the wild has not yet been observed. The five stages of their development were originally thought to be differe ...
. Although they spend most of their lives in fresh water, usually in rivers, streams, or estuaries, they go to spawn and die in the Sargasso Sea
The Sargasso Sea () is a region of the Atlantic Ocean bounded by four currents forming an ocean gyre. Unlike all other regions called seas, it is the only one without land boundaries. It is distinguished from other parts of the Atlantic Oc ...
. Here is where European eels are experiencing the effects of acidification in one of their key life stages.
Fish embryos and larvae are usually more sensitive to pH changes than adults, as organs for pH regulation are not full developed. Because of this, European eel embryos are more vulnerable to changes in pH in the Sargasso Sea. A study of the European Eel in the Sargasso Sea was conducted in 2021 to analyze the specific effects of ocean acidification on embryos. The study found that exposure to predicted end-of-century ocean pCO2 conditions may affect normal development of this species in nature during sensitive early life history stages with limited physiological response capacities, while extreme acidification would negatively influence embryonic survival and development under hatchery conditions.
Compounded effects of acidification, warming and deoxygenation
 There is a substantial body of research showing that a combination of ocean acidification and elevated
There is a substantial body of research showing that a combination of ocean acidification and elevated ocean temperature
The ocean temperature plays a crucial role in the global climate system, ocean currents and for marine habitats. It varies depending on depth, geographical location and season. Not only does the temperature differ in seawater, so does the salin ...
have a compounded effect on marine life and the ocean environment. This effect far exceeds the individual harmful impact of either. In addition, ocean warming, along with increased productivity of phytoplankton from higher CO2 levels exacerbates ocean deoxygenation
Ocean deoxygenation is the reduction of the oxygen content in different parts of the ocean due to human activities. There are two areas where this occurs. Firstly, it occurs in coastal zones where eutrophication has driven some quite rapid (in ...
. Deoxygenation of ocean waters is an additional stressor on marine organisms that increases ocean stratification
Ocean stratification is the natural separation of an ocean's water into horizontal layers by Density of water, density. This is generally stable stratification, because warm water floats on top of cold water, and heating is mostly from the sun, whi ...
therefore limiting nutrients over time and reducing biological gradients.
Meta analyses have quantified the direction and magnitude of the harmful effects of combined ocean acidification, warming and deoxygenation on the ocean. These meta-analyses have been further tested by mesocosm
thumb , Diagram of a small form closed system mesocosm.
A mesocosm (''meso-'' or 'medium' and ''-cosm'' 'world') is any outdoor experimental system that examines the natural environment under controlled conditions. In this way mesocosm studi ...
studies that simulated the interaction of these stressors and found a catastrophic effect on the marine food web: thermal stress more than negates any primary producer to herbivore increase in productivity from elevated .
Impacts on the economy and societies
The increase of ocean acidity decelerates the rate of calcification in salt water, leading to smaller and slower growing coral reef
A coral reef is an underwater ecosystem characterized by reef-building corals. Reefs are formed of colonies of coral polyps held together by calcium carbonate. Most coral reefs are built from stony corals, whose polyps cluster in group ...
s which supports approximately 25% of marine life.
Fishing and tourism industry
The threat of acidification includes a decline in commercial fisheries
Commercial fishing is the activity of catching fish and other seafood for commercial profit, mostly from wild fisheries. It provides a large quantity of food to many countries around the world, but those who practice it as an industry must often p ...
and the coast-based tourism industry
Tourism is travel for pleasure, and the Commerce, commercial activity of providing and supporting such travel. World Tourism Organization, UN Tourism defines tourism more generally, in terms which go "beyond the common perception of tourism as ...
. Several ocean goods and services are likely to be undermined by future ocean acidification potentially affecting the livelihoods of some 400 to 800 million people, depending upon the greenhouse gas emission scenario.food chains
''Food Chains'' is a 2014 American documentary film about agricultural labor in the United States directed by Sanjay Rawal. It was the Recipient of the 2015 James Beard Foundation Award for Special/Documentary.James Beard Foundation/ The 2015 Book, ...
linked with the oceans.
Arctic
In the Arctic, commercial fisheries are threatened because acidification harms calcifying organisms which form the base of the Arctic food webs (pteropods and brittle stars, see above). Acidification threatens Arctic food webs from the base up. Arctic food webs are considered simple, meaning there are few steps in the food chain from small organisms to larger predators. For example, pteropods are "a key prey item of a number of higher predators – larger plankton, fish, seabirds, whales". Both pteropods and sea stars serve as a substantial food source and their removal from the simple food web would pose a serious threat to the whole ecosystem. The effects on the calcifying organisms at the base of the food webs could potentially destroy fisheries.
United Kingdom commercial fisheries
The shellfish industry is an important part of the United Kingdom economy.
US commercial fisheries
 The value of fish caught from US commercial fisheries in 2007 was valued at $3.8 billion and of that 73% was derived from calcifiers and their direct predators. Other organisms are directly harmed as a result of acidification. For example, decrease in the growth of marine calcifiers such as the
The value of fish caught from US commercial fisheries in 2007 was valued at $3.8 billion and of that 73% was derived from calcifiers and their direct predators. Other organisms are directly harmed as a result of acidification. For example, decrease in the growth of marine calcifiers such as the American lobster
The American lobster (''Homarus americanus'') is a species of lobster found on the Atlantic Ocean, Atlantic coast of North America, chiefly from Labrador to New Jersey. It is also known as Atlantic lobster, Canadian lobster, true lobster, norther ...
, ocean quahog
The ocean quahog (''Arctica islandica'') is a species of edible clam, a marine (ocean), marine bivalve mollusc, mollusk in the family Arcticidae. This species is native to the North Atlantic Ocean, and it is harvested commercially as a food sou ...
, and scallop
Scallop () is a common name that encompasses various species of marine bivalve molluscs in the taxonomic family Pectinidae, the scallops. However, the common name "scallop" is also sometimes applied to species in other closely related famili ...
s means there is less shellfish meat available for sale and consumption. Red king crab fisheries are also at a serious threat because crabs are also calcifiers. Baby red king crab when exposed to increased acidification levels experienced 100% mortality after 95 days. In 2006, red king crab accounted for 23% of the total guideline harvest levels and a serious decline in red crab population would threaten the crab harvesting industry.
Possible responses
Climate change mitigation
Reducing carbon dioxide emissions (i.e. climate change mitigation
Climate change mitigation (or decarbonisation) is action to limit the greenhouse gases in the atmosphere that cause climate change. Climate change mitigation actions include energy conservation, conserving energy and Fossil fuel phase-out, repl ...
measures) is the only solution that addresses the root cause of ocean acidification. For example, some mitigation measures focus on carbon dioxide removal
Carbon dioxide removal (CDR) is a process in which carbon dioxide () is removed from the atmosphere by deliberate human activities and durably stored in geological, terrestrial, or ocean reservoirs, or in products.IPCC, 2021:Annex VII: Glossar ...
(CDR) from the atmosphere (e.g. direct air capture (DAC), bioenergy with carbon capture and storage (BECCS)). These would also slow the rate of acidification.
Approaches that remove carbon dioxide from the ocean include ocean nutrient fertilization, artificial upwelling/downwelling, seaweed farming
Seaweed farming or kelp farming is the practice of aquaculture, cultivating and harvesting seaweed. In its simplest form farmers gather from natural beds, while at the other extreme farmers fully control the crop's biological life cycle, life c ...
, ecosystem recovery, ocean alkalinity enhancement, enhanced weathering
Enhanced weathering, also termed ocean alkalinity enhancement when proposed for carbon credit systems, is a process that aims to accelerate the natural weathering by spreading finely ground silicate rock, such as basalt, onto surfaces which speeds ...
and electrochemical processes.[IPCC (2022]
Chapter 12: Cross sectoral perspectives
i
Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change
, Cambridge University Press, Cambridge, United Kingdom and New York, NY, US All of these methods use the ocean to remove from the atmosphere to store it in the ocean. These methods could assist with mitigation but they can have side-effects on marine life. The research field for all CDR methods has grown a lot since 2019.[IPCC (2022]
Technical Summary
. I
Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change
, Cambridge University Press, Cambridge, United Kingdom and New York, NY, US Their costs are in the order of per ton of . For example, enhanced weathering
Enhanced weathering, also termed ocean alkalinity enhancement when proposed for carbon credit systems, is a process that aims to accelerate the natural weathering by spreading finely ground silicate rock, such as basalt, onto surfaces which speeds ...
could remove of per year. This technology comes with a cost of per ton of .
Carbon removal technologies which add alkalinity
Some carbon removal
Carbon dioxide removal (CDR) is a process in which carbon dioxide () is removed from the atmosphere by deliberate human activities and durably stored in geological, terrestrial, or ocean reservoirs, or in products.IPCC, 2021:Annex VII: Glossar ...
techniques add alkalinity to the ocean and therefore immediately buffer pH changes which might help the organisms in the region that the extra alkalinity is added to. The two technologies that fall into this category are ocean alkalinity enhancement and electrochemical methods.technology readiness level
Technology readiness levels (TRLs) are a method for estimating the maturity of technologies during the acquisition phase of a program. TRLs enable consistent and uniform discussions of technical maturity across different types of technology. TR ...
.
Ocean alkalinity enhancement
Ocean alkalinity enhancement (OAE) is a proposed "carbon dioxide removal (CDR) method that involves deposition of alkaline minerals or their dissociation products at the ocean surface".[IPCC, 2021]
Annex VII: Glossary
atthews, J.B.R., V. Möller, R. van Diemen, J.S. Fuglestvedt, V. Masson-Delmotte, C. Méndez, S. Semenov, A. Reisinger (eds.) I
Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change
[Masson-Delmotte, V., P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, US The process would increase surface total alkalinity. It would work to increase ocean absorption of . The process involves increasing the amount of bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial bioche ...
(HCO3-) through accelerated weathering (enhanced weathering
Enhanced weathering, also termed ocean alkalinity enhancement when proposed for carbon credit systems, is a process that aims to accelerate the natural weathering by spreading finely ground silicate rock, such as basalt, onto surfaces which speeds ...
) of rocks (silicate
A silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is also used ...
, limestone
Limestone is a type of carbonate rock, carbonate sedimentary rock which is the main source of the material Lime (material), lime. It is composed mostly of the minerals calcite and aragonite, which are different Polymorphism (materials science) ...
and quicklime
Calcium oxide (formula: Ca O), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term '' lime'' connotes calcium-containin ...
).Industrial Revolution
The Industrial Revolution, sometimes divided into the First Industrial Revolution and Second Industrial Revolution, was a transitional period of the global economy toward more widespread, efficient and stable manufacturing processes, succee ...
.
Experimental materials include limestone, brucite
Brucite is the mineral form of magnesium hydroxide, with the chemical formula Magnesium, Mg(hydroxyl, OH)2. It is a common alteration product of periclase in marble; a low-temperature hydrothermal Vein (geology), vein mineral in metamorphosed li ...
, olivine
The mineral olivine () is a magnesium iron Silicate minerals, silicate with the chemical formula . It is a type of Nesosilicates, nesosilicate or orthosilicate. The primary component of the Earth's upper mantle (Earth), upper mantle, it is a com ...
and alkaline solutions. Another approach is to use electricity to raise alkalinity during desalination to capture waterborne CO2.
Electrochemical methods
Electrochemical methods, or electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses Direct current, direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of c ...
, can strip carbon dioxide directly from seawater.hydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
.
However, implementation of electrolysis for carbon capture is expensive and the energy consumed for the process is high compared to other CDR techniques.
Policies and goals
Global policies
As awareness about ocean acidification grows, policies geared towards increasing monitoring efforts of ocean acidification have been drafted. Previously in 2015, ocean scientist Jean-Pierre Gattuso
Jean-Pierre Gattuso () (born 14 December 1958 in Antibes) is a French ocean scientist conducting research globally, from the pole to the tropics and from nearshore to the open ocean. His research addresses the biology of reef-building corals, th ...
had remarked that "The ocean has been minimally considered at previous climate negotiations. Our study provides compelling arguments for a radical change at the UN conference (in Paris) on climate change".
UN Ocean Decade
The UN Ocean Decade has a program called "Ocean acidification research for sustainability". It was proposed by the Global Ocean Acidification Observing Network (GOA-ON) and its partners, and has been formally endorsed as a program of the UN Decade of Ocean Science for Sustainable Development
Earth system governance (or earth systems governance) is a broad area of scholarly inquiry that builds on earlier notions of environmental policy and nature conservation, but puts these into the broader context of human-induced transformations of ...
. The OARS program builds on the work of GOA-ON and has the following aims: to further develop the science of ocean acidification; to increase observations of ocean chemistry changes; to identify the impacts on marine ecosystems on local and global scales; and to provide decision makers with the information needed to mitigate and adapt to ocean acidification.
Global Climate Indicators
The importance of ocean acidification is reflected in its inclusion as one of seven Global Climate Indicators. These Indicators are a set of parameters that describe the changing climate without reducing climate change to only rising temperature. The Indicators include key information for the most relevant domains of climate change: temperature and energy, atmospheric composition, ocean and water as well as the cryosphere. The Global Climate Indicators have been identified by scientists and communication specialists in a process led by Global Climate Observing System
The Global Climate Observing System (GCOS) was established in 1992 as an outcome of the Second World Climate Conference, to ensure that the observations and information needed to address climate-related issues are obtained and made available to ...
(GCOS). The Indicators have been endorsed by the World Meteorological Organization
The World Meteorological Organization (WMO) is a List of specialized agencies of the United Nations, specialized agency of the United Nations responsible for promoting international cooperation on atmospheric science, climatology, hydrology an ...
(WMO). They form the basis of the annual WMO Statement of the State of the Global Climate, which is submitted to the Conference of Parties (COP) of the United Nations Framework Convention on Climate Change
The United Nations Framework Convention on Climate Change (UNFCCC) is the UN process for negotiating an agreement to limit dangerous climate change. It is an international treaty among countries to combat "dangerous human interference with th ...
(UNFCCC). Additionally, the Copernicus Climate Change Service
The Copernicus Climate Change Service (abbreviated as C3S) is one of the six thematic services provided by the European Union's Copernicus Programme. The Copernicus Programme is managed by the European Commission and the C3S is implemented by th ...
(C3S) of the European Commission uses the Indicators for their annual "European State of the Climate".
Sustainable Development Goal 14
In 2015, the United Nations adopted the 2030 Agenda and a set of 17 Sustainable Development Goals (SDG), including a goal dedicated to the ocean, Sustainable Development Goal 14
Sustainable Development Goal 14 (Goal 14 or SDG 14) is about "Life below water" and is one of the 17 Sustainable Development Goals established by the United Nations in 2015. The official wording is to "Conserve and sustainably use the oceans, seas ...
,[United Nations (2017) Resolution adopted by the General Assembly on 6 July 2017, :File:A RES 71 313 E.pdf, Work of the Statistical Commission pertaining to the 2030 Agenda for Sustainable Development]
A/RES/71/313
) This target has one indicator: Indicator 14.3.1 which calls for the "Average marine acidity ( pH) measured at agreed suite of representative sampling stations".
Policies at country level
United States
In the United States, the Federal Ocean Acidification Research And Monitoring Act of 2009 supports government coordination, such as the National Oceanic and Atmospheric Administration, National Oceanic Atmospheric Administration's (NOAA) "Ocean Acidification Program". In 2015, USEPA denied a citizens petition that asked EPA to regulate under the Toxic Substances Control Act of 1976 in order to mitigate ocean acidification. In the denial, the EPA said that risks from ocean acidification were being "more efficiently and effectively addressed" under domestic actions, e.g., under the Presidential Climate Action Plan, and that multiple avenues are being pursued to work with and in other nations to reduce emissions and deforestation and promote clean energy and energy efficiency.
History
Research into the phenomenon of ocean acidification, as well as awareness raising about the problem, has been going on for several decades. The fundamental research really began with the creation of the pH scale by Danish people, Danish chemist Søren Peder Lauritz Sørensen in 1909.[ Two other publications appeared in 1909, one in French and one in Danish.] By around the 1950s the massive role of the ocean in absorbing fossil fuel CO2 was known to specialists, but not appreciated by the greater scientific community.[ Text was copied from this source, which is available under a creativecommons:by/3.0/, Creative Commons Attribution 3.0 Unported License]
In the early 1970s questions over the long-term impact of the accumulation of fossil fuel CO2 in the sea were already arising around the world and causing strong debate. Researchers commented on the accumulation of fossil CO2 in the atmosphere and sea and drew attention to the possible impacts on marine life. By the mid-1990s, the likely impact of CO2 levels rising so high with the inevitable changes in pH and carbonate ion became a concern of scientists studying the fate of coral reefs.[, Secretariat: TWAS (the Academy of Sciences for the Developing World), Trieste, Italy.] The statement also stressed the importance to "Reinvigorate action to reduce stressors, such as overfishing and marine pollution, pollution, on marine ecosystem
Marine ecosystems are the largest of Earth's aquatic ecosystems and exist in Saline water, waters that have a high salt content. These systems contrast with freshwater ecosystems, which have a lower salt content. Marine waters cover more than 7 ...
s to increase resilience to ocean acidification".
For example, research in 2010 found that in the 15-year period 1995–2010 alone, acidity had increased 6 percent in the upper 100 meters of the Pacific Ocean from Hawaii to Alaska.
See also
*
*
*
*
*
References
External links
Global Ocean Acidification Observing Network (GOA-ON)
United Nations Decade of Ocean Science for Sustainable Development (2021–2030)
US NOAA Ocean Acidification Program
{{DEFAULTSORT:Ocean Acidification
Aquatic ecology
Biological oceanography
Carbon
Chemical oceanography
Fisheries science
Geochemistry
Oceanography
Effects of climate change
Environmental impact by effect
Articles containing video clips
Oceanographical terminology
 Ocean acidification is the ongoing decrease in the pH of the Earth's
Ocean acidification is the ongoing decrease in the pH of the Earth's  In 2021, atmospheric carbon dioxide (CO2) levels of around 415 ppm were around 50% higher than preindustrial concentrations. Text was copied from this source, which is available under
In 2021, atmospheric carbon dioxide (CO2) levels of around 415 ppm were around 50% higher than preindustrial concentrations. Text was copied from this source, which is available under  The
The  Between 1950 and 2020, the average pH value of the ocean surface is estimated to have decreased from approximately 8.15 to 8.05. This represents an increase of around 26% in hydrogen ion concentration in the world's oceans (the pH scale is logarithmic, so a change of one in pH unit is equivalent to a tenfold change in hydrogen ion concentration). For example, in the 15-year period 1995–2010 alone, acidity has increased 6 percent in the upper 100 meters of the Pacific Ocean from Hawaii to Alaska.
The
Between 1950 and 2020, the average pH value of the ocean surface is estimated to have decreased from approximately 8.15 to 8.05. This represents an increase of around 26% in hydrogen ion concentration in the world's oceans (the pH scale is logarithmic, so a change of one in pH unit is equivalent to a tenfold change in hydrogen ion concentration). For example, in the 15-year period 1995–2010 alone, acidity has increased 6 percent in the upper 100 meters of the Pacific Ocean from Hawaii to Alaska.
The  Importantly, the rate of change in ocean acidification is much higher than in the geological past. This faster change prevents organisms from gradually adapting, and prevents climate cycle feedbacks from kicking in to mitigate ocean acidification. Ocean acidification is now on a path to reach lower pH levels than at any other point in the last 300 million years. The rate of ocean acidification (i.e. the rate of change in pH value) is also estimated to be unprecedented over that same time scale. These expected changes are considered unprecedented in the geological record. In combination with other ocean
Importantly, the rate of change in ocean acidification is much higher than in the geological past. This faster change prevents organisms from gradually adapting, and prevents climate cycle feedbacks from kicking in to mitigate ocean acidification. Ocean acidification is now on a path to reach lower pH levels than at any other point in the last 300 million years. The rate of ocean acidification (i.e. the rate of change in pH value) is also estimated to be unprecedented over that same time scale. These expected changes are considered unprecedented in the geological record. In combination with other ocean 

 The value of fish caught from US commercial fisheries in 2007 was valued at $3.8 billion and of that 73% was derived from calcifiers and their direct predators. Other organisms are directly harmed as a result of acidification. For example, decrease in the growth of marine calcifiers such as the
The value of fish caught from US commercial fisheries in 2007 was valued at $3.8 billion and of that 73% was derived from calcifiers and their direct predators. Other organisms are directly harmed as a result of acidification. For example, decrease in the growth of marine calcifiers such as the