Azo Pigments on:
[Wikipedia]
[Google]
[Amazon]
Azo dyes are
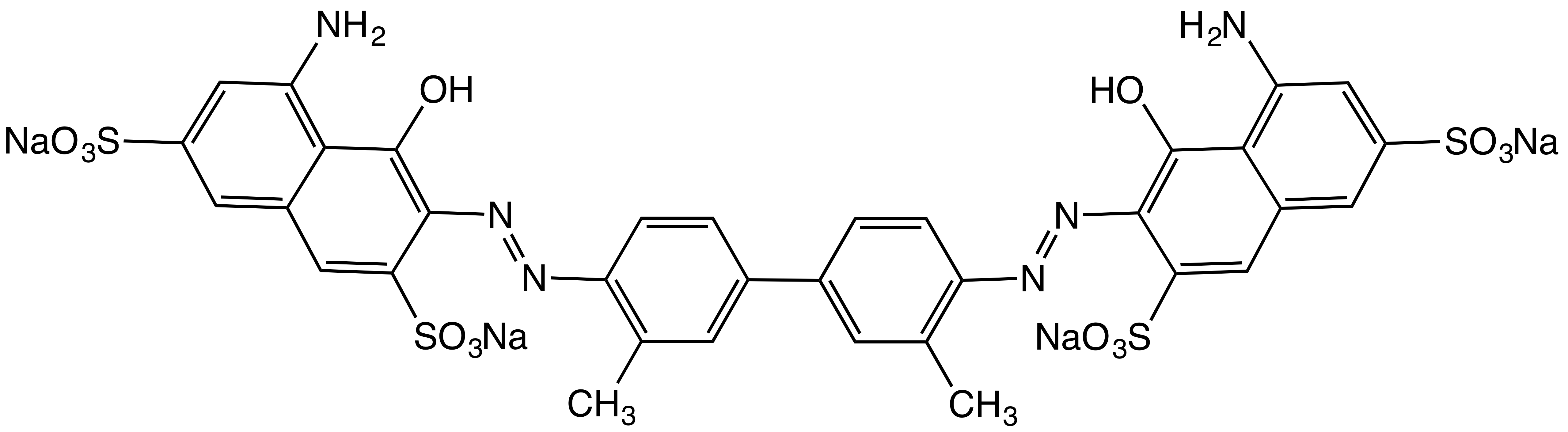
 Azo dyes are solids. Most are salts, the colored component being the anion usually, although some cationic azo dyes are known. The anionic character of most dyes arises from the presence of 1-3 sulfonic acid groups, which are fully ionized at the pH of the dyed article:
:RSO3H → RSO3− + H+
Most proteins are cationic, thus dyeing of leather and wool corresponds to an
Azo dyes are solids. Most are salts, the colored component being the anion usually, although some cationic azo dyes are known. The anionic character of most dyes arises from the presence of 1-3 sulfonic acid groups, which are fully ionized at the pH of the dyed article:
:RSO3H → RSO3− + H+
Most proteins are cationic, thus dyeing of leather and wool corresponds to an
 A unique property of azo dyes is their ability to undergo reversible photoisomerization between ''trans'' and ''cis'' configurations. In the trans isomer, the aromatic rings (or other substituents) are on opposite sides of the double bond, resulting in a more extended and linear conformation. Upon exposure to ultraviolet (UV) light, typically at 365 nm, the molecule absorbs energy and converts to the cis isomer, where the substituents are on the same side of the N=N bond, leading to a bent or kinked structure.
This process is reversible: the ''cis'' form can thermally relax back to the ''trans'' form over time, or be actively reverted using visible light irradiation (commonly around 450–500 nm), depending on the substituents and molecular environment.
A unique property of azo dyes is their ability to undergo reversible photoisomerization between ''trans'' and ''cis'' configurations. In the trans isomer, the aromatic rings (or other substituents) are on opposite sides of the double bond, resulting in a more extended and linear conformation. Upon exposure to ultraviolet (UV) light, typically at 365 nm, the molecule absorbs energy and converts to the cis isomer, where the substituents are on the same side of the N=N bond, leading to a bent or kinked structure.
This process is reversible: the ''cis'' form can thermally relax back to the ''trans'' form over time, or be actively reverted using visible light irradiation (commonly around 450–500 nm), depending on the substituents and molecular environment.
File:NaO3SazoNaphthNH2.png, Direct Brown 78
File:NaO3SazoOMeMeNH2.png
File:NaO3SMeOazoOMeMeNH2.png
File:Pontamine sky blue.svg,
 Azo pigments are important in a variety of plastics, rubbers, and
Azo pigments are important in a variety of plastics, rubbers, and
organic compounds
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
bearing the functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
R−N=N−R′, in which R and R′ are usually aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
and substituted aryl groups. They are a commercially important family of azo compound
Azo compounds are organic compounds bearing the functional group diazenyl (, in which R and R′ can be either aryl or alkyl groups).
IUPAC defines azo compounds as: "Derivatives of diazene (diimide), , wherein both hydrogens are substituted ...
s, i.e. compounds containing the C−N=N−C linkage. Azo dyes are synthetic dye
Juan de Guillebon, better known by his stage name DyE, is a French musician. He is known for the music video of the single "Fantasy
Fantasy is a genre of speculative fiction that involves supernatural or Magic (supernatural), magical ele ...
s and do not occur naturally. Most azo dyes contain only one azo group but there are some that contain two or three azo groups, called "diazo dyes" and "triazo dyes" respectively. Azo dyes comprise 60–70% of all dyes used in food
Food is any substance consumed by an organism for Nutrient, nutritional support. Food is usually of plant, animal, or Fungus, fungal origin and contains essential nutrients such as carbohydrates, fats, protein (nutrient), proteins, vitamins, ...
and textile
Textile is an Hyponymy and hypernymy, umbrella term that includes various Fiber, fiber-based materials, including fibers, yarns, Staple (textiles)#Filament fiber, filaments, Thread (yarn), threads, and different types of #Fabric, fabric. ...
industries. Azo dyes are widely used to treat textiles
Textile is an Hyponymy and hypernymy, umbrella term that includes various Fiber, fiber-based materials, including fibers, yarns, Staple (textiles)#Filament fiber, filaments, Thread (yarn), threads, and different types of #Fabric, fabric. ...
, leather articles, and some foods. Chemically related derivatives of azo dyes include azo pigments, which are insoluble in water and other solvents.
Classes
Many kinds of azo dyes are known, and several classification systems exist. Some classes include disperse dyes,metal-complex dyes
Metal-complex dyes are a family of dyes that contain metals coordinated to the organic portion. Many azo dyes, especially those derived from naphthols, form metal complexes by complexation of one of the azo nitrogen centers. The insertion of the ...
, reactive dye
In a reactive dye, a chromophore (an atom or group whose presence is responsible for the colour of a compound) contains a substituent that reacts with the substrate. Reactive dyes have good fastness properties owing to the covalent bonding that o ...
s, and substantive dyes. Also called direct dyes, substantive dyes are employed for cellulose-based textiles, which includes cotton. The dyes bind to the textile by non-electrostatic forces. In another classification, azo dyes can be classified according to the number of azo groups.

Physical properties, structure, and bonding
As a consequence of π-delocalization
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref>
The term delocalization is general and can have slightly diff ...
, aryl azo compounds have vivid colors, especially reds, oranges, and yellows. An example is Disperse Orange 1
Disperse Orange 1, or 4-anilino-4'-nitroazobenzene, is an azo dye. Commercial samples contain approximately 25% dye by weight, with the remaining mass consisting of NaCl and other salts.
This dye is useful in conducting experiments with flash ...
. Some azo compounds, e.g., methyl orange
Methyl orange is a pH indicator frequently used in titration because of its clear and distinct color variance at different pH values. Methyl orange shows red color in acidic medium and yellow color in basic medium. Because it changes color at the p ...
, are used as acid-base indicators
A pH indicator is a halochromic chemical compound added in small amounts to a solution so the pH (acidity or basicity) of the solution can be determined visually or spectroscopically by changes in absorption and/or emission properties. Hence, ...
. Most DVD-R
DVD recordable and DVD rewritable are a collection of optical disc formats that can be written to by a DVD recorder and by computers using a DVD writer. The "recordable" discs are write-once read-many (WORM) media, where as "rewritable" discs a ...
/ +R and some CD-R
CD-R (Compact disc-recordable) is a digital media, digital optical disc data storage device, storage format. A CD-R disc is a compact disc that can only be Write once read many, written once and read arbitrarily many times.
CD-R discs (CD-Rs) ...
discs use blue azo dye as the recording layer.
 Azo dyes are solids. Most are salts, the colored component being the anion usually, although some cationic azo dyes are known. The anionic character of most dyes arises from the presence of 1-3 sulfonic acid groups, which are fully ionized at the pH of the dyed article:
:RSO3H → RSO3− + H+
Most proteins are cationic, thus dyeing of leather and wool corresponds to an
Azo dyes are solids. Most are salts, the colored component being the anion usually, although some cationic azo dyes are known. The anionic character of most dyes arises from the presence of 1-3 sulfonic acid groups, which are fully ionized at the pH of the dyed article:
:RSO3H → RSO3− + H+
Most proteins are cationic, thus dyeing of leather and wool corresponds to an ion exchange
Ion exchange is a reversible interchange of one species of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid. Ion exchange is used in softening or demineralizing of water, purification of ch ...
reaction. The anionic dye adheres to these articles through electrostatic forces. Cationic azo dyes typically contain quaternary ammonium
In organic chemistry, quaternary ammonium cations, also known as quats, are positively-charged polyatomic ions of the structure , where R is an alkyl group, an aryl group or organyl group. Unlike the ammonium ion () and the primary, secondary, o ...
centers.
Reversible photoisomerization
 A unique property of azo dyes is their ability to undergo reversible photoisomerization between ''trans'' and ''cis'' configurations. In the trans isomer, the aromatic rings (or other substituents) are on opposite sides of the double bond, resulting in a more extended and linear conformation. Upon exposure to ultraviolet (UV) light, typically at 365 nm, the molecule absorbs energy and converts to the cis isomer, where the substituents are on the same side of the N=N bond, leading to a bent or kinked structure.
This process is reversible: the ''cis'' form can thermally relax back to the ''trans'' form over time, or be actively reverted using visible light irradiation (commonly around 450–500 nm), depending on the substituents and molecular environment.
A unique property of azo dyes is their ability to undergo reversible photoisomerization between ''trans'' and ''cis'' configurations. In the trans isomer, the aromatic rings (or other substituents) are on opposite sides of the double bond, resulting in a more extended and linear conformation. Upon exposure to ultraviolet (UV) light, typically at 365 nm, the molecule absorbs energy and converts to the cis isomer, where the substituents are on the same side of the N=N bond, leading to a bent or kinked structure.
This process is reversible: the ''cis'' form can thermally relax back to the ''trans'' form over time, or be actively reverted using visible light irradiation (commonly around 450–500 nm), depending on the substituents and molecular environment.
Preparation
Most azo dyes are prepared byazo coupling
In organic chemistry, an azo coupling is an organic reaction, reaction between a diazonium compound () and another aromatic compound that produces an azo compound (). In this electrophilic aromatic substitution reaction, the aryldiazonium cation ...
, which entails an electrophilic substitution reaction
Electrophilic substitution reactions are chemical reactions in which an electrophile displaces a functional group in a compound, which is typically, but not always, aromatic. Aromatic substitution reactions are characteristic of aromatic compounds ...
of an aryl diazonium cation with another compound, the coupling partner. Generally, coupling partners are other aromatic compounds with electron-donating groups:
: + Ar′H → ArN=NAr′ + H+
In practice, acetoacetanilide
Acetoacetanilide is an organic compound with the formula CH3C(O)CH2C(O)NHC6H5. It is the acetoacetamide derivative of aniline. It is a white solid that is poorly soluble in water. This chemical and many related compounds (prepared from various ...
s are widely used as coupling partners:
: + Ar′NHC(O)CH2C(O)Me → ArN=NCH(C(O)Me)(C(O)NHAr′) + H+
Azo dyes are also prepared by the condensation of nitrated aromatic compounds with anilines followed by reduction of the resulting azoxy
In chemistry, azoxy compounds are a group of organic compounds sharing a common functional group with the general structure . They are considered Amine oxide, N-oxides of azo compounds. Azoxy compounds are 1,3-dipoles and 1,3-dipolar cycloadditio ...
intermediate:
:ArNO2 + Ar′NH2 → ArN(O)=NAr′ + H2O
:ArN(O)=NAr′ + C6H12O6 → ArN=NAr′ + C6H10O6 + H2O
For textile dying, a typical nitro coupling partner would be disodium 4,4′-dinitrostilbene-2,2′-disulfonate. Typical aniline partners are shown below. Since anilines are prepared from nitro compounds, some azo dyes are produced by partial reduction of aromatic nitro compounds.
Many azo dyes are produced by reactions from pre-existing azo compounds. Typical reactions include metal complexation and acylation.
Direct Blue 1
Direct Blue 1 is an organic compound that is one of many azo dye
Azo dyes are organic compounds bearing the functional group R−N=N−R′, in which R and R′ are usually aryl and substituted aryl groups. They are a commercially important f ...
File:BasicRed18.png, Basic Red 18
Basic or BASIC may refer to:
Science and technology
* BASIC, a computer programming language
* Basic (chemistry), having the properties of a base
* Basic access authentication, in HTTP
Entertainment
* ''Basic'' (film), a 2003 film
* Basic, one ...
, a cationic azo dye
Azo pigments
Azo pigments are similar in chemical structure to azo dyes, but they lack solubilizing groups.K. Hunger. W. Herbst "Pigments, Organic" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim, 2012. Many so-called azo pigments are not strictly classifiable as azo compounds since they exist as keto hydrazide tautomers, which lack the -N=N- linkage.paint
Paint is a material or mixture that, when applied to a solid material and allowed to dry, adds a film-like layer. As art, this is used to create an image or images known as a painting. Paint can be made in many colors and types. Most paints are ...
s (including artist's paints). They have excellent coloring properties, mainly in the yellow to red range, as well as good lightfastness
Lightfastness is a property of a colourant such as dye or pigment that describes its resistance to fading when exposed to light. Dyes and pigments are used for example for dyeing of fabrics, plastics or other materials and manufacturing paints o ...
. The lightfastness depends not only on the properties of the organic azo compound, but also on the way they have been absorbed on the pigment carrier.
Biodegradation
In order for dyes to be useful, they must possess a high degree of chemical and photolytic stability. As a result of this stability,photolysis
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by absorption of light or photons. It is defined as the interaction of one or more photons wi ...
is not considered to be a degradation pathway for azo dyes. In order to prolong the lifetime of products dyed with azo dyes, it is essential to ensure stability against microbial attack, and tests have shown that azo dyes biodegrade negligibly in short term tests under aerobic conditions. Under anaerobic conditions, however, discoloration may be observed as a consequence of biodegradation.
Safety and regulation
Many azo pigments are non-toxic, although some, such asdinitroaniline
Dinitroanilines are a class of chemical compounds with the chemical formula C6H5N3O4. They are derived from both aniline and dinitrobenzenes. There are six isomers: 2,3-dinitroaniline, 2,4-dinitroaniline, 2,5-dinitroaniline, 2,6-dinitroaniline, 3 ...
orange, ortho-nitroaniline
2-Nitroaniline is an organic compound with the formula H2NC6H4NO2. It is a derivative of aniline, carrying a nitro functional group in position 2. It is mainly used as a precursor to o-phenylenediamine.
Synthesis
2-Nitroaniline is prepared commer ...
orange, or pigment orange 1, 2, and 5 are mutagen
In genetics, a mutagen is a physical or chemical agent that permanently changes genetic material, usually DNA, in an organism and thus increases the frequency of mutations above the natural background level. As many mutations can cause cancer in ...
ic and carcinogenic
A carcinogen () is any agent that promotes the development of cancer. Carcinogens can include synthetic chemicals, naturally occurring substances, physical agents such as ionizing and non-ionizing radiation, and Biological agent, biologic agent ...
.
Azo dyes derived from benzidine
Benzidine (trivial name), also called 1,1'-biphenyl-4,4'-diamine (systematic name), is an organic compound with the chemical formula, formula (C6H4NH2)2. It is an aromatic amine. It is a component of a test for cyanide. Related derivatives are ...
are carcinogen
A carcinogen () is any agent that promotes the development of cancer. Carcinogens can include synthetic chemicals, naturally occurring substances, physical agents such as ionizing and non-ionizing radiation, and biologic agents such as viruse ...
s; exposure to them has classically been associated with bladder cancer
Bladder cancer is the abnormal growth of cells in the bladder. These cells can grow to form a tumor, which eventually spreads, damaging the bladder and other organs. Most people with bladder cancer are diagnosed after noticing blood in thei ...
. Accordingly, the production of benzidine azo dyes was discontinued in the 1980s in many western countries.
European regulation
Certain azo dyes degrade under reductive conditions to release any of a group of defined aromatic amines. Since September 2003, the European Union has banned the manufacture or sale of consumer goods which contain the listed amines. Since only a small number of dyes produced those amines, relatively few products were actually affected.See also
*Azo coupling
In organic chemistry, an azo coupling is an organic reaction, reaction between a diazonium compound () and another aromatic compound that produces an azo compound (). In this electrophilic aromatic substitution reaction, the aryldiazonium cation ...
* Ponceau 4R
Ponceau 4R (known by more than 100 synonyms,Abbey J, et at. Colorants. pp 459-465 in Encyclopedia of Food Safety, Vol 2: Hazards and Diseases. Eds, Motarjemi Y et al. Academic Press, 2013. including as C.I. 16255,FDA. 9 November 2008Food and ...
* Ponceau S
Ponceau S, Acid Red 112, or C.I. 27195 (systematic name: 3-hydroxy-4-(2-sulfo-4- -sulfophenylazohenylazo)-2,7-naphthalenedisulfonic acid sodium salt) is a sodium salt of a diazo dye of a light red color, that may be used to prepare a stain fo ...
* Glycoazodyes Glycoazodyes (or GADs) are a family of "naturalised" synthetic dyes, so called because they are the conjugation of common commercial azo dyes with sugar through a "linker". This principle is summarised in the scheme below.
Generations, Structur ...
References
{{reflist Organic pigments