Auwers Synthesis on:
[Wikipedia]
[Google]
[Amazon]
The Auwers synthesis is a series of  The first step in this procedure is an acid catalyzed
The first step in this procedure is an acid catalyzed
organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, mechanistic organ ...
s forming a flavonol
Flavonols are a class of flavonoids that have the 3-hydroxyflavone backbone (IUPAC name: 3-hydroxy-2-phenylchromen-4-one). Their diversity stems from the different positions of the phenolic –OH groups. They are distinct from flavanols (with ...
from a coumarone. This reaction was first reported by Karl von Auwers
Karl Friedrich von Auwers (September 16, 1863 – May 3, 1939) was a German chemist, and was the academic adviser of both Karl Ziegler and Georg Wittig at the University of Marburg.
Life
Karl Friedrich von Auwers was born the son of the renowned ...
in 1908.K. v. Auwers, E. Auffenberg, "Über Cumaranone und Hydrindone", '' Ber. Dtsch. Chem. Ges.'', 52, 92-113 (1919) ().
aldol condensation
An aldol condensation is a condensation reaction in organic chemistry in which two carbonyl moieties (of aldehydes or ketones) react to form a β-hydroxyaldehyde or β-hydroxyketone (an aldol reaction), and this is then followed by dehydration t ...
between benzaldehyde
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is among the simplest aromatic aldehydes and one of the most industrially useful.
It is a colorless liquid with a characteristic almond-li ...
and a 3-cyclooxapentanone to an o-hydroxychalcone. Bromination
In chemistry, halogenation is a chemical reaction which introduces one or more halogens into a chemical compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs ...
of the alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
group gives a dibromo-adduct which rearranges to the flavonol by reaction with potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash.
Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which utili ...
.
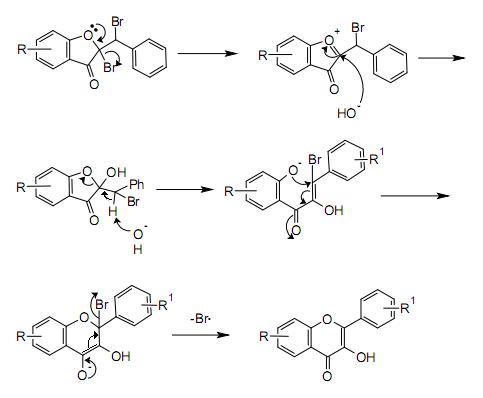
Mechanism
A possible mechanism for the rearrangement step is shown below:
See also
* Algar–Flynn–Oyamada reaction *Allan–Robinson reaction
The Allan–Robinson reaction is the chemical reaction of o-hydroxyaryl ketones with aromatic anhydrides to form flavones (or isoflavones).
If aliphatic anhydrides are used, coumarins can also be formed. (See Kostanecki acylation.)
:
Mechanism ...
References
{{Organic reactions Name reactions Oxygen heterocycle forming reactions Ring expansion reactions Carbon-carbon bond forming reactions