atomic diffusion on:
[Wikipedia]
[Google]
[Amazon]
 In chemical physics, atomic diffusion is a
In chemical physics, atomic diffusion is a
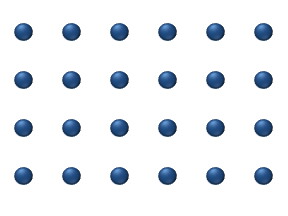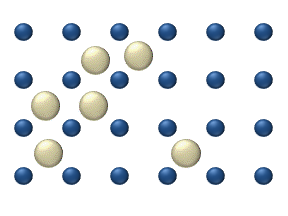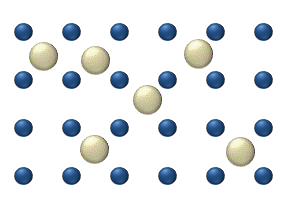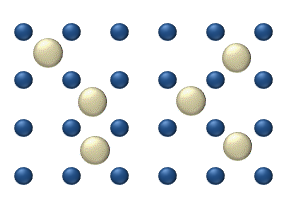 In the crystal solid state, diffusion within the crystal lattice occurs by either interstitial or substitutional mechanisms and is referred to as lattice diffusion. In interstitial lattice diffusion, a diffusant (such as C in an iron alloy), will diffuse in between the lattice structure of another crystalline element. In substitutional lattice diffusion ( self-diffusion for example), the atom can only move by substituting place with another atom. Substitutional lattice diffusion is often contingent upon the availability of point vacancies throughout the crystal lattice. Diffusing particles migrate from point vacancy to point vacancy by the rapid, essentially random jumping about
(
In the crystal solid state, diffusion within the crystal lattice occurs by either interstitial or substitutional mechanisms and is referred to as lattice diffusion. In interstitial lattice diffusion, a diffusant (such as C in an iron alloy), will diffuse in between the lattice structure of another crystalline element. In substitutional lattice diffusion ( self-diffusion for example), the atom can only move by substituting place with another atom. Substitutional lattice diffusion is often contingent upon the availability of point vacancies throughout the crystal lattice. Diffusing particles migrate from point vacancy to point vacancy by the rapid, essentially random jumping about
(
Classical and nanoscale diffusion (with figures and animations)
{{DEFAULTSORT:Atomic Diffusion Diffusion Crystallographic defects
diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical p ...
process whereby the random, thermally-activated movement of atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
s in a solid
Solid is a state of matter where molecules are closely packed and can not slide past each other. Solids resist compression, expansion, or external forces that would alter its shape, with the degree to which they are resisted dependent upon the ...
results in the net transport of atoms. For example, helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is ...
atoms inside a balloon can diffuse through the wall of the balloon and escape, resulting in the balloon slowly deflating. Other air molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
s (e.g. oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
, nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
) have lower mobilities and thus diffuse more slowly through the balloon wall. There is a concentration gradient in the balloon wall, because the balloon was initially filled with helium, and thus there is plenty of helium on the inside, but there is relatively little helium on the outside (helium is not a major component of air). The rate of transport is governed by the diffusivity and the concentration gradient.
In crystals
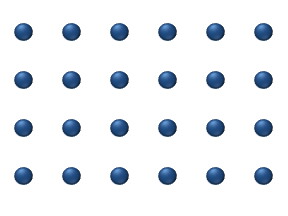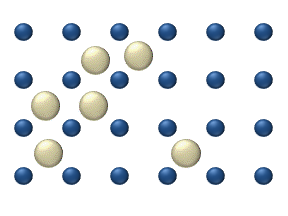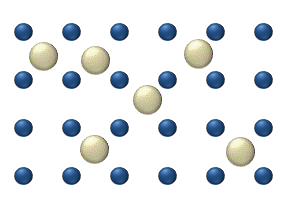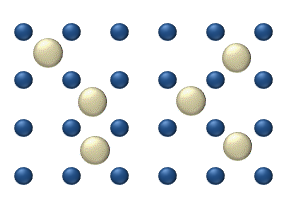 In the crystal solid state, diffusion within the crystal lattice occurs by either interstitial or substitutional mechanisms and is referred to as lattice diffusion. In interstitial lattice diffusion, a diffusant (such as C in an iron alloy), will diffuse in between the lattice structure of another crystalline element. In substitutional lattice diffusion ( self-diffusion for example), the atom can only move by substituting place with another atom. Substitutional lattice diffusion is often contingent upon the availability of point vacancies throughout the crystal lattice. Diffusing particles migrate from point vacancy to point vacancy by the rapid, essentially random jumping about
(
In the crystal solid state, diffusion within the crystal lattice occurs by either interstitial or substitutional mechanisms and is referred to as lattice diffusion. In interstitial lattice diffusion, a diffusant (such as C in an iron alloy), will diffuse in between the lattice structure of another crystalline element. In substitutional lattice diffusion ( self-diffusion for example), the atom can only move by substituting place with another atom. Substitutional lattice diffusion is often contingent upon the availability of point vacancies throughout the crystal lattice. Diffusing particles migrate from point vacancy to point vacancy by the rapid, essentially random jumping about
(jump diffusion
Jump diffusion is a stochastic process that involves jump process, jumps and diffusion process, diffusion. It has important applications in magnetic reconnection, coronal mass ejections, condensed matter physics, and pattern theory and computationa ...
).
Since the prevalence of point vacancies increases in accordance with the Arrhenius equation
In physical chemistry, the Arrhenius equation is a formula for the temperature dependence of reaction rates. The equation was proposed by Svante Arrhenius in 1889, based on the work of Dutch chemist Jacobus Henricus van 't Hoff who had noted in 188 ...
, the rate of crystal solid state diffusion increases with temperature.
For a single atom in a defect-free crystal, the movement can be described by the "random walk
In mathematics, a random walk, sometimes known as a drunkard's walk, is a stochastic process that describes a path that consists of a succession of random steps on some Space (mathematics), mathematical space.
An elementary example of a rand ...
" model. In 3-dimensions it can be shown that after jumps of length the atom will have moved, on average, a distance of:
:
If the jump frequency is given by (in jumps per second) and time is given by , then is proportional to the square root of :
:
Diffusion in polycrystalline materials can involve short circuit diffusion mechanisms. For example, along the grain boundaries and certain crystalline defects such as dislocations there is more open space, thereby allowing for a lower activation energy for diffusion. Atomic diffusion in polycrystalline materials is therefore often modeled using an effective diffusion coefficient, which is a combination of lattice, and grain boundary diffusion coefficients. In general, surface diffusion
Surface diffusion is a general process involving the motion of adatoms, molecules, and atomic clusters ( adparticles) at solid material surfaces.Oura, Lifshits, Saranin, Zotov, and Katayama 2003, p. 325 The process can generally be thought of in ...
occurs much faster than grain boundary diffusion, and grain boundary diffusion occurs much faster than lattice diffusion.
See also
*Kirkendall effect
The Kirkendall effect is the motion of the interface between two metals that occurs due to the difference in diffusion rates of the metal atoms. The effect can be observed, for example, by placing insoluble markers at the interface between a pure m ...
* Mass diffusivity
References
External links
Classical and nanoscale diffusion (with figures and animations)
{{DEFAULTSORT:Atomic Diffusion Diffusion Crystallographic defects