Atoms are the basic particles of the
chemical element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its ...
s. An atom consists of a
nucleus
Nucleus (: nuclei) is a Latin word for the seed inside a fruit. It most often refers to:
*Atomic nucleus, the very dense central region of an atom
*Cell nucleus, a central organelle of a eukaryotic cell, containing most of the cell's DNA
Nucleu ...
of
proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
s and generally
neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
s, surrounded by an electromagnetically bound swarm of
electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s. The chemical elements are distinguished from each other by the number of protons that are in their atoms. For example, any atom that contains 11 protons is
sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the peri ...
, and any atom that contains 29 protons is
copper
Copper is a chemical element; it has symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orang ...
. Atoms with the same number of protons but a different number of neutrons are called
isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
s of the same element.
Atoms are extremely small, typically around 100
picometer
The picometre (international spelling as used by the International Bureau of Weights and Measures; SI symbol: pm) or picometer ( American spelling) is a unit of length in the International System of Units (SI), equal to , or one trillionth o ...
s across. A human hair is about a million
carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
atoms wide. Atoms are smaller than the shortest wavelength of visible light, which means humans cannot see atoms with conventional microscopes. They are so small that accurately predicting their behavior using
classical physics
Classical physics refers to physics theories that are non-quantum or both non-quantum and non-relativistic, depending on the context. In historical discussions, ''classical physics'' refers to pre-1900 physics, while '' modern physics'' refers to ...
is not possible due to
quantum effects
Quantum mechanics is the fundamental physical theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Addison-Wesley, 1989, It is the foundation of a ...
.
More than 99.94% of an atom's
mass
Mass is an Intrinsic and extrinsic properties, intrinsic property of a physical body, body. It was traditionally believed to be related to the physical quantity, quantity of matter in a body, until the discovery of the atom and particle physi ...
is in the nucleus. Protons have a positive
electric charge
Electric charge (symbol ''q'', sometimes ''Q'') is a physical property of matter that causes it to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative''. Like charges repel each other and ...
and neutrons have no charge, so the nucleus is positively charged. The electrons are negatively charged, and this opposing charge is what binds them to the nucleus. If the numbers of
proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
s and electrons are equal, as they normally are, then the atom is electrically neutral as a whole. If an atom has more electrons than protons, then it has an overall negative charge and is called a negative
ion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
(or anion). Conversely, if it has more protons than electrons, it has a positive charge and is called a positive ion (or cation).
The electrons of an atom are attracted to the protons in an atomic nucleus by the
electromagnetic force
In physics, electromagnetism is an interaction that occurs between particles with electric charge via electromagnetic fields. The electromagnetic force is one of the four fundamental forces of nature. It is the dominant force in the interac ...
. The protons and neutrons in the nucleus are attracted to each other by the
nuclear force
The nuclear force (or nucleon–nucleon interaction, residual strong force, or, historically, strong nuclear force) is a force that acts between hadrons, most commonly observed between protons and neutrons of atoms. Neutrons and protons, both ...
. This force is usually stronger than the electromagnetic force that repels the positively charged protons from one another. Under certain circumstances, the repelling electromagnetic force becomes stronger than the nuclear force. In this case, the nucleus
splits and leaves behind different elements. This is a form of
nuclear decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
.
Atoms can attach to one or more other atoms by
chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons a ...
s to form
chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
s such as
molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
s or
crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macros ...
s. The ability of atoms to attach and detach from each other is responsible for most of the physical changes observed in nature.
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
is the science that studies these changes.
History of atomic theory
In philosophy
The basic idea that matter is made up of tiny indivisible particles is an old idea that appeared in many ancient cultures. The word ''atom'' is derived from the
ancient Greek
Ancient Greek (, ; ) includes the forms of the Greek language used in ancient Greece and the classical antiquity, ancient world from around 1500 BC to 300 BC. It is often roughly divided into the following periods: Mycenaean Greek (), Greek ...
word ''atomos'', which means "uncuttable". But this ancient idea was based in philosophical reasoning rather than scientific reasoning. Modern atomic theory is not based on these old concepts. In the early 19th century, the scientist
John Dalton
John Dalton (; 5 or 6 September 1766 – 27 July 1844) was an English chemist, physicist and meteorologist. He introduced the atomic theory into chemistry. He also researched Color blindness, colour blindness; as a result, the umbrella term ...
found evidence that matter really is composed of discrete units, and so applied the word ''atom'' to those units.
Dalton's law of multiple proportions

In the early 1800s, John Dalton compiled experimental data gathered by him and other scientists and discovered a pattern now known as the "
law of multiple proportions
In chemistry, the law of multiple proportions states that in compounds which contain two particular chemical elements, the amount of Element A per measure of Element B will differ across these compounds by ratios of small whole numbers. For inst ...
". He noticed that in any group of chemical compounds which all contain two particular chemical elements, the amount of Element A per measure of Element B will differ across these compounds by ratios of small
whole numbers
An integer is the number zero (0), a positive natural number (1, 2, 3, ...), or the negation of a positive natural number (−1, −2, −3, ...). The negations or additive inverses of the positive natural numbers are referred to as negative in ...
. This pattern suggested that each element combines with other elements in multiples of a basic unit of weight, with each element having a unit of unique weight. Dalton decided to call these units "atoms".
For example, there are two types of
tin oxide Tin oxide may refer to:
* Tin(II) oxide (stannous oxide), a black powder with the formula SnO
* Tin(IV) oxide
Tin(IV) oxide, also known as stannic oxide, is the inorganic compound with the formula SnO2. The mineral form of SnO2 is called cassite ...
: one is a grey powder that is 88.1% tin and 11.9%
oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
, and the other is a white powder that is 78.7% tin and 21.3% oxygen. Adjusting these figures, in the grey powder there is about 13.5 g of oxygen for every 100 g of tin, and in the white powder there is about 27 g of oxygen for every 100 g of tin. 13.5 and 27 form a ratio of 1:2. Dalton concluded that in the grey oxide there is one atom of oxygen for every atom of tin, and in the white oxide there are two atoms of oxygen for every atom of tin (
SnO Sno or SNO may refer to:
People
* Evander Sno (born 1987), Dutch football coach and former player
* Shaquill Sno (born 1996), Dutch footballer
Other uses
* " Snö", a song by Iranian-Swedish singer-songwriter Laleh
* Sakon Nakhon Airport, T ...
and
SnO2).
Dalton also analyzed
iron oxide
An iron oxide is a chemical compound composed of iron and oxygen. Several iron oxides are recognized. Often they are non-stoichiometric. Ferric oxyhydroxides are a related class of compounds, perhaps the best known of which is rust.
Iron ...
s. There is one type of iron oxide that is a black powder which is 78.1% iron and 21.9% oxygen; and there is another iron oxide that is a red powder which is 70.4% iron and 29.6% oxygen. Adjusting these figures, in the black powder there is about 28 g of oxygen for every 100 g of iron, and in the red powder there is about 42 g of oxygen for every 100 g of iron. 28 and 42 form a ratio of 2:3. Dalton concluded that in these oxides, for every two atoms of iron, there are two or three atoms of oxygen respectively. These substances are known today as
iron(II) oxide
Iron(II) oxide or ferrous oxide is the inorganic compound with the formula FeO. Its mineral form is known as wüstite. One of several iron oxides, it is a black-colored powder that is sometimes confused with rust, the latter of which consists ...
and
iron(III) oxide
Iron(III) oxide or ferric oxide is the inorganic compound with the formula . It occurs in nature as the mineral hematite, which serves as the primary source of iron for the steel industry. It is also known as red iron oxide, especially when use ...
, and their formulas are FeO and Fe
2O
3 respectively. Iron(II) oxide's formula is normally written as FeO, but since it is a crystalline substance we could alternately write it as Fe
2O
2, and when we contrast that with Fe
2O
3, the 2:3 ratio for the oxygen is plain to see.
As a final example:
nitrous oxide
Nitrous oxide (dinitrogen oxide or dinitrogen monoxide), commonly known as laughing gas, nitrous, or factitious air, among others, is a chemical compound, an Nitrogen oxide, oxide of nitrogen with the Chemical formula, formula . At room te ...
is 63.3%
nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
and 36.7% oxygen,
nitric oxide
Nitric oxide (nitrogen oxide, nitrogen monooxide, or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes den ...
is 44.05% nitrogen and 55.95% oxygen, and
nitrogen dioxide
Nitrogen dioxide is a chemical compound with the formula . One of several nitrogen oxides, nitrogen dioxide is a reddish-brown gas. It is a paramagnetic, bent molecule with C2v point group symmetry. Industrially, is an intermediate in the s ...
is 29.5% nitrogen and 70.5% oxygen. Adjusting these figures, in nitrous oxide there is 80 g of oxygen for every 140 g of nitrogen, in nitric oxide there is about 160 g of oxygen for every 140 g of nitrogen, and in nitrogen dioxide there is 320 g of oxygen for every 140 g of nitrogen. 80, 160, and 320 form a ratio of 1:2:4. The respective formulas for these oxides are
N2O,
NO, and
NO2.
Discovery of the electron
In 1897,
J. J. Thomson
Sir Joseph John Thomson (18 December 1856 – 30 August 1940) was an English physicist who received the Nobel Prize in Physics in 1906 "in recognition of the great merits of his theoretical and experimental investigations on the conduction of ...
discovered that
cathode ray
Cathode rays are streams of electrons observed in discharge tubes. If an evacuated glass tube is equipped with two electrodes and a voltage is applied, glass behind the positive electrode is observed to glow, due to electrons emitted from the c ...
s can be deflected by electric and magnetic fields, which meant that cathode rays are not a form of light but made of electrically charged particles, and their charge was negative given the direction the particles were deflected in. He measured these particles to be 1,700 times lighter than
hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
(the lightest atom). He called these new particles ''corpuscles'' but they were later renamed ''
electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s'' since these are the particles that carry electricity. Thomson also showed that electrons were identical to particles given off by
photoelectric
The photoelectric effect is the emission of electrons from a material caused by electromagnetic radiation such as ultraviolet light. Electrons emitted in this manner are called photoelectrons. The phenomenon is studied in condensed matter physic ...
and radioactive materials.
Thomson explained that an electric current is the passing of electrons from one atom to the next, and when there was no current the electrons embedded themselves in the atoms. This in turn meant that atoms were not indivisible as scientists thought. The atom was composed of electrons whose negative charge was balanced out by some source of positive charge to create an electrically neutral atom. Ions, Thomson explained, must be atoms which have an excess or shortage of electrons.
Discovery of the nucleus

The electrons in the atom logically had to be balanced out by a commensurate amount of positive charge, but Thomson had no idea where this positive charge came from, so he tentatively proposed that it was everywhere in the atom, the atom being in the shape of a sphere. This was the mathematically simplest hypothesis to fit the available evidence, or lack thereof. Following from this, Thomson imagined that the balance of electrostatic forces would distribute the electrons throughout the sphere in a more or less even manner. Thomson's model is popularly known as the
plum pudding model
The plum pudding model is an obsolete scientific model of the atom. It was first proposed by J. J. Thomson in 1904 following his discovery of the electron in 1897, and was rendered obsolete by Ernest Rutherford's discovery of the atomic nucleus i ...
, though neither Thomson nor his colleagues used this analogy.
Thomson's model was incomplete, it was unable to predict any other properties of the elements such as
emission spectra
The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to electrons making a transition from a high energy state to a lower energy state. The photon energy of the ...
and
valencies. It was soon rendered obsolete by the discovery of the
atomic nucleus
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford at the Department_of_Physics_and_Astronomy,_University_of_Manchester , University of Manchester ...
.
Between 1908 and 1913,
Ernest Rutherford
Ernest Rutherford, 1st Baron Rutherford of Nelson (30 August 1871 – 19 October 1937) was a New Zealand physicist who was a pioneering researcher in both Atomic physics, atomic and nuclear physics. He has been described as "the father of nu ...
and his colleagues
Hans Geiger
Johannes Wilhelm Geiger ( , ; ; 30 September 1882 – 24 September 1945) was a German nuclear physicist. He is known as the inventor of the Geiger counter, a device used to detect ionizing radiation, and for carrying out the Rutherford scatt ...
and
Ernest Marsden
Sir Ernest Marsden (19 February 1889 – 15 December 1970) was an English-New Zealand physicist. He is recognised internationally for his contributions to science while working under Ernest Rutherford, which led to the discovery of new theories ...
performed a series of experiments in which they bombarded thin foils of metal with a beam of
alpha particles
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay but may also be produce ...
. They did this to measure the scattering patterns of the alpha particles. They spotted a small number of alpha particles being deflected by angles greater than 90°. This shouldn't have been possible according to the Thomson model of the atom, whose charges were too diffuse to produce a sufficiently strong electric field. The deflections should have all been negligible. Rutherford proposed that the positive charge of the atom is concentrated in a tiny volume at the center of the atom and that the electrons surround this nucleus in a diffuse cloud. This nucleus carried almost all of the atom's mass. Only such an intense concentration of charge, anchored by its high mass, could produce an electric field that could deflect the alpha particles so strongly.
[ Heilbron (2003). ''Ernest Rutherford and the Explosion of Atoms'', pp. 64–68]
Bohr model
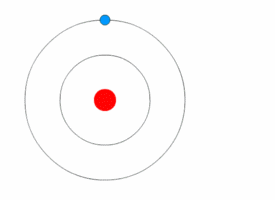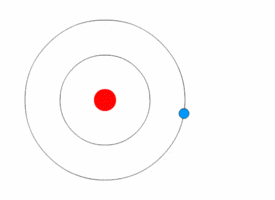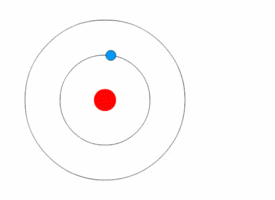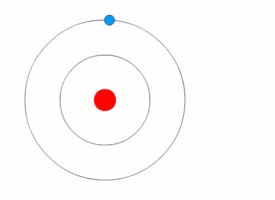
A problem in classical mechanics is that an accelerating charged particle radiates
electromagnetic radiation
In physics, electromagnetic radiation (EMR) is a self-propagating wave of the electromagnetic field that carries momentum and radiant energy through space. It encompasses a broad spectrum, classified by frequency or its inverse, wavelength ...
, causing the particle to lose
kinetic energy
In physics, the kinetic energy of an object is the form of energy that it possesses due to its motion.
In classical mechanics, the kinetic energy of a non-rotating object of mass ''m'' traveling at a speed ''v'' is \fracmv^2.Resnick, Rober ...
. Circular motion counts as acceleration, which means that an electron orbiting a central charge should spiral down into that nucleus as it loses speed. In 1913, the physicist
Niels Bohr
Niels Henrik David Bohr (, ; ; 7 October 1885 – 18 November 1962) was a Danish theoretical physicist who made foundational contributions to understanding atomic structure and old quantum theory, quantum theory, for which he received the No ...
proposed a new model in which the electrons of an atom were assumed to orbit the nucleus but could only do so in a finite set of orbits, and could jump between these orbits only in discrete changes of energy corresponding to absorption or radiation of a
photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
.
This quantization was used to explain why the electrons' orbits are stable and why elements absorb and emit electromagnetic radiation in discrete spectra.
Bohr's model could only predict the emission spectra of hydrogen, not atoms with more than one electron.
Discovery of protons and neutrons
Back in 1815,
William Prout
William Prout Fellow of the Royal Society, FRS (; 15 January 1785 – 9 April 1850) was an English chemist, physician, and natural theologian. He is remembered today mainly for what is called Prout's hypothesis.
Biography
Prout was born in H ...
observed that the atomic weights of many elements were multiples of hydrogen's atomic weight, which is in fact true for all of them if one takes
isotopes
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), but ...
into account. In 1898,
J. J. Thomson
Sir Joseph John Thomson (18 December 1856 – 30 August 1940) was an English physicist who received the Nobel Prize in Physics in 1906 "in recognition of the great merits of his theoretical and experimental investigations on the conduction of ...
found that the positive charge of a hydrogen ion is equal to the negative charge of an electron, and these were then the smallest known charged particles. Thomson later found that the positive charge in an atom is a positive multiple of an electron's negative charge. In 1913,
Henry Moseley
Henry Gwyn Jeffreys Moseley (; 23 November 1887 – 10 August 1915) was an English physicist, whose contribution to the science of physics was the justification from physical laws of the previous empirical and chemical concept of the atomic numb ...
discovered that the frequencies of X-ray emissions from an
excited atom were a mathematical function of its
atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
and hydrogen's nuclear charge. In 1919,
Rutherford bombarded
nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
gas with
alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay but may also be produce ...
s and detected
hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
ions being emitted from the gas, and concluded that they were produced by alpha particles hitting and splitting the nuclei of the nitrogen atoms.
These observations led Rutherford to conclude that the hydrogen nucleus is a singular particle with a positive charge equal to the electron's negative charge. He named this particle "
proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
" in 1920. The number of protons in an atom (which Rutherford called the "
atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
") was found to be equal to the element's ordinal number on the
periodic table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other s ...
and therefore provided a simple and clear-cut way of distinguishing the elements from each other. The atomic weight of each element is higher than its proton number, so Rutherford hypothesized that the surplus weight was carried by unknown particles with no electric charge and a mass equal to that of the proton.
In 1928,
Walter Bothe observed that
beryllium
Beryllium is a chemical element; it has Symbol (chemistry), symbol Be and atomic number 4. It is a steel-gray, hard, strong, lightweight and brittle alkaline earth metal. It is a divalent element that occurs naturally only in combination with ...
emitted a highly penetrating, electrically neutral radiation when bombarded with alpha particles. It was later discovered that this radiation could knock hydrogen atoms out of
paraffin wax
Paraffin wax (or petroleum wax) is a soft colorless solid derived from petroleum, coal, or oil shale that consists of a mixture of hydrocarbon molecules containing between 20 and 40 carbon atoms. It is solid at room temperature and melting poi ...
. Initially it was thought to be high-energy
gamma radiation
A gamma ray, also known as gamma radiation (symbol ), is a penetrating form of electromagnetic radiation arising from high energy interactions like the radioactive decay of atomic nuclei or astronomical events like solar flares. It consists o ...
, since gamma radiation had a similar effect on electrons in metals, but
James Chadwick
Sir James Chadwick (20 October 1891 – 24 July 1974) was an English nuclear physicist who received the Nobel Prize in Physics in 1935 for his discovery of the neutron. In 1941, he wrote the final draft of the MAUD Report, which inspired t ...
found that the
ionization
Ionization or ionisation is the process by which an atom or a molecule acquires a negative or positive Electric charge, charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged at ...
effect was too strong for it to be due to electromagnetic radiation, so long as energy and momentum were conserved in the interaction. In 1932, Chadwick exposed various elements, such as hydrogen and nitrogen, to the mysterious "beryllium radiation", and by measuring the energies of the recoiling charged particles, he deduced that the radiation was actually composed of electrically neutral particles which could not be massless like the gamma ray, but instead were required to have a mass similar to that of a proton. Chadwick now claimed these particles as Rutherford's neutrons.
The current consensus model

In 1925,
Werner Heisenberg
Werner Karl Heisenberg (; ; 5 December 1901 – 1 February 1976) was a German theoretical physicist, one of the main pioneers of the theory of quantum mechanics and a principal scientist in the German nuclear program during World War II.
He pub ...
published the first consistent mathematical formulation of quantum mechanics (
matrix mechanics
Matrix mechanics is a formulation of quantum mechanics created by Werner Heisenberg, Max Born, and Pascual Jordan in 1925. It was the first conceptually autonomous and logically consistent formulation of quantum mechanics. Its account of quantum ...
).
One year earlier,
Louis de Broglie
Louis Victor Pierre Raymond, 7th Duc de Broglie (15 August 1892 – 19 March 1987) was a French theoretical physicist and aristocrat known for his contributions to quantum theory. In his 1924 PhD thesis, he postulated the wave nature of elec ...
had proposed that all particles behave like waves to some extent, and in 1926
Erwin Schrödinger
Erwin Rudolf Josef Alexander Schrödinger ( ; ; 12 August 1887 – 4 January 1961), sometimes written as or , was an Austrian-Irish theoretical physicist who developed fundamental results in quantum field theory, quantum theory. In particul ...
used this idea to develop the
Schrödinger equation
The Schrödinger equation is a partial differential equation that governs the wave function of a non-relativistic quantum-mechanical system. Its discovery was a significant landmark in the development of quantum mechanics. It is named after E ...
, which describes electrons as three-dimensional
waveform
In electronics, acoustics, and related fields, the waveform of a signal is the shape of its Graph of a function, graph as a function of time, independent of its time and Magnitude (mathematics), magnitude Scale (ratio), scales and of any dis ...
s rather than points in space. A consequence of using waveforms to describe particles is that it is mathematically impossible to obtain precise values for both the
position
Position often refers to:
* Position (geometry), the spatial location (rather than orientation) of an entity
* Position, a job or occupation
Position may also refer to:
Games and recreation
* Position (poker), location relative to the dealer
* ...
and
momentum
In Newtonian mechanics, momentum (: momenta or momentums; more specifically linear momentum or translational momentum) is the product of the mass and velocity of an object. It is a vector quantity, possessing a magnitude and a direction. ...
of a particle at a given point in time. This became known as the
uncertainty principle
The uncertainty principle, also known as Heisenberg's indeterminacy principle, is a fundamental concept in quantum mechanics. It states that there is a limit to the precision with which certain pairs of physical properties, such as position a ...
, formulated by Werner Heisenberg in 1927.
In this concept, for a given accuracy in measuring a position one could only obtain a range of probable values for momentum, and vice versa. Thus, the planetary model of the atom was discarded in favor of one that described
atomic orbital
In quantum mechanics, an atomic orbital () is a Function (mathematics), function describing the location and Matter wave, wave-like behavior of an electron in an atom. This function describes an electron's Charge density, charge distribution a ...
zones around the nucleus where a given electron is most likely to be found.
This model was able to explain observations of atomic behavior that previous models could not, such as certain structural and
spectral
''Spectral'' is a 2016 Hungarian-American military science fiction action film co-written and directed by Nic Mathieu. Written with Ian Fried & George Nolfi, the film stars James Badge Dale as DARPA research scientist Mark Clyne, with Max Marti ...
patterns of atoms larger than hydrogen.
Structure
Subatomic particles
Though the word ''atom'' originally denoted a particle that cannot be cut into smaller particles, in modern scientific usage the atom is composed of various
subatomic particle
In physics, a subatomic particle is a particle smaller than an atom. According to the Standard Model of particle physics, a subatomic particle can be either a composite particle, which is composed of other particles (for example, a baryon, lik ...
s. The constituent particles of an atom are the
electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
, the
proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
, and the
neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
.
The electron is the least massive of these particles by four orders of magnitude at , with a negative
electrical charge
Electricity is the set of physical phenomena associated with the presence and motion of matter possessing an electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described by Maxwel ...
and a size that is too small to be measured using available techniques. It was the lightest particle with a positive rest mass measured, until the discovery of
neutrino
A neutrino ( ; denoted by the Greek letter ) is an elementary particle that interacts via the weak interaction and gravity. The neutrino is so named because it is electrically neutral and because its rest mass is so small ('' -ino'') that i ...
mass. Under ordinary conditions, electrons are bound to the positively charged nucleus by the attraction created from opposite electric charges. If an atom has more or fewer electrons than its atomic number, then it becomes respectively negatively or positively charged as a whole; a charged atom is called an
ion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
. Electrons have been known since the late 19th century, mostly thanks to
J.J. Thomson
Sir Joseph John Thomson (18 December 1856 – 30 August 1940) was an English physicist who received the Nobel Prize in Physics in 1906 "in recognition of the great merits of his theoretical and experimental investigations on the conduction of ...
; see
history of subatomic physics for details.
Protons have a positive charge and a mass of . The number of protons in an atom is called its
atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
.
Ernest Rutherford
Ernest Rutherford, 1st Baron Rutherford of Nelson (30 August 1871 – 19 October 1937) was a New Zealand physicist who was a pioneering researcher in both Atomic physics, atomic and nuclear physics. He has been described as "the father of nu ...
(1919) observed that nitrogen under alpha-particle bombardment ejects what appeared to be hydrogen nuclei. By 1920 he had accepted that the hydrogen nucleus is a distinct particle within the atom and named it
proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
.
Neutrons have no electrical charge and have a mass of .
[Mohr, P.J.; Taylor, B.N. and Newell, D.B. (2014)]
"The 2014 CODATA Recommended Values of the Fundamental Physical Constants"
(Web Version 7.0). The database was developed by J. Baker, M. Douma, and S. Kotochigova. (2014). National Institute of Standards and Technology, Gaithersburg, Maryland 20899. Neutrons are the heaviest of the three constituent particles, but their mass can be reduced by the
nuclear binding energy
Nuclear binding energy in experimental physics is the minimum energy that is required to disassemble the nucleus of an atom into its constituent protons and neutrons, known collectively as nucleons. The binding energy for stable nuclei is alwa ...
. Neutrons and protons (collectively known as
nucleon
In physics and chemistry, a nucleon is either a proton or a neutron, considered in its role as a component of an atomic nucleus. The number of nucleons in a nucleus defines the atom's mass number.
Until the 1960s, nucleons were thought to be ele ...
s) have comparable dimensions—on the order of —although the 'surface' of these particles is not sharply defined. The neutron was discovered in 1932 by the English physicist
James Chadwick
Sir James Chadwick (20 October 1891 – 24 July 1974) was an English nuclear physicist who received the Nobel Prize in Physics in 1935 for his discovery of the neutron. In 1941, he wrote the final draft of the MAUD Report, which inspired t ...
.
In the
Standard Model
The Standard Model of particle physics is the Scientific theory, theory describing three of the four known fundamental forces (electromagnetism, electromagnetic, weak interaction, weak and strong interactions – excluding gravity) in the unive ...
of physics, electrons are truly elementary particles with no internal structure, whereas protons and neutrons are composite particles composed of
elementary particle
In particle physics, an elementary particle or fundamental particle is a subatomic particle that is not composed of other particles. The Standard Model presently recognizes seventeen distinct particles—twelve fermions and five bosons. As a c ...
s called
quark
A quark () is a type of elementary particle and a fundamental constituent of matter. Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nucleus, atomic nuclei ...
s. There are two types of quarks in atoms, each having a fractional electric charge. Protons are composed of two
up quark
The up quark or u quark (symbol: u) is the lightest of all quarks, a type of elementary particle, and a significant constituent of matter. It, along with the down quark, forms the neutrons (one up quark, two down quarks) and protons (two up quark ...
s (each with charge +) and one
down quark
The down quark (symbol: d) is a type of elementary particle, and a major constituent of matter. The down quark is the second-lightest of all quarks, and combines with other quarks to form composite particles called hadrons. Down quarks are most ...
(with a charge of −). Neutrons consist of one up quark and two down quarks. This distinction accounts for the difference in mass and charge between the two particles.
The quarks are held together by the
strong interaction
In nuclear physics and particle physics, the strong interaction, also called the strong force or strong nuclear force, is one of the four known fundamental interaction, fundamental interactions. It confines Quark, quarks into proton, protons, n ...
(or strong force), which is mediated by
gluon
A gluon ( ) is a type of Massless particle, massless elementary particle that mediates the strong interaction between quarks, acting as the exchange particle for the interaction. Gluons are massless vector bosons, thereby having a Spin (physi ...
s. The protons and neutrons, in turn, are held to each other in the nucleus by the
nuclear force
The nuclear force (or nucleon–nucleon interaction, residual strong force, or, historically, strong nuclear force) is a force that acts between hadrons, most commonly observed between protons and neutrons of atoms. Neutrons and protons, both ...
, which is a residuum of the strong force that has somewhat different range-properties (see the article on the nuclear force for more). The gluon is a member of the family of
gauge boson
In particle physics, a gauge boson is a bosonic elementary particle that acts as the force carrier for elementary fermions. Elementary particles whose interactions are described by a gauge theory interact with each other by the exchange of gauge ...
s, which are elementary particles that mediate physical forces.
Nucleus

All the bound protons and neutrons in an atom make up a tiny
atomic nucleus
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford at the Department_of_Physics_and_Astronomy,_University_of_Manchester , University of Manchester ...
, and are collectively called
nucleon
In physics and chemistry, a nucleon is either a proton or a neutron, considered in its role as a component of an atomic nucleus. The number of nucleons in a nucleus defines the atom's mass number.
Until the 1960s, nucleons were thought to be ele ...
s. The radius of a nucleus is approximately equal to
 In the early 1800s, John Dalton compiled experimental data gathered by him and other scientists and discovered a pattern now known as the "
In the early 1800s, John Dalton compiled experimental data gathered by him and other scientists and discovered a pattern now known as the " The electrons in the atom logically had to be balanced out by a commensurate amount of positive charge, but Thomson had no idea where this positive charge came from, so he tentatively proposed that it was everywhere in the atom, the atom being in the shape of a sphere. This was the mathematically simplest hypothesis to fit the available evidence, or lack thereof. Following from this, Thomson imagined that the balance of electrostatic forces would distribute the electrons throughout the sphere in a more or less even manner. Thomson's model is popularly known as the
The electrons in the atom logically had to be balanced out by a commensurate amount of positive charge, but Thomson had no idea where this positive charge came from, so he tentatively proposed that it was everywhere in the atom, the atom being in the shape of a sphere. This was the mathematically simplest hypothesis to fit the available evidence, or lack thereof. Following from this, Thomson imagined that the balance of electrostatic forces would distribute the electrons throughout the sphere in a more or less even manner. Thomson's model is popularly known as the  A problem in classical mechanics is that an accelerating charged particle radiates
A problem in classical mechanics is that an accelerating charged particle radiates  In 1925,
In 1925,  All the bound protons and neutrons in an atom make up a tiny
All the bound protons and neutrons in an atom make up a tiny  In the early 1800s, John Dalton compiled experimental data gathered by him and other scientists and discovered a pattern now known as the "
In the early 1800s, John Dalton compiled experimental data gathered by him and other scientists and discovered a pattern now known as the " A problem in classical mechanics is that an accelerating charged particle radiates
A problem in classical mechanics is that an accelerating charged particle radiates