Atom (sparse Signal Analysis) on:
[Wikipedia]
[Google]
[Amazon]
An atom is a particle that consists of a
 In the early 1800s, the English chemist
In the early 1800s, the English chemist
 In 1897,
In 1897,
 In 1913, the physicist
In 1913, the physicist
"The 2014 CODATA Recommended Values of the Fundamental Physical Constants"
(Web Version 7.0). The database was developed by J. Baker, M. Douma, and S. Kotochigova. (2014). National Institute of Standards and Technology, Gaithersburg, Maryland 20899. Neutrons are the heaviest of the three constituent particles, but their mass can be reduced by the
 All the bound protons and neutrons in an atom make up a tiny
All the bound protons and neutrons in an atom make up a tiny  The number of protons and neutrons in the atomic nucleus can be modified, although this can require very high energies because of the strong force. Nuclear fusion occurs when multiple atomic particles join to form a heavier nucleus, such as through the energetic collision of two nuclei. For example, at the core of the Sun protons require energies of 3 to 10 keV to overcome their mutual repulsion—the coulomb barrier—and fuse together into a single nucleus. Nuclear fission is the opposite process, causing a nucleus to split into two smaller nuclei—usually through radioactive decay. The nucleus can also be modified through bombardment by high energy subatomic particles or photons. If this modifies the number of protons in a nucleus, the atom changes to a different chemical element.
If the mass of the nucleus following a fusion reaction is less than the sum of the masses of the separate particles, then the difference between these two values can be emitted as a type of usable energy (such as a gamma ray, or the kinetic energy of a beta particle), as described by
The number of protons and neutrons in the atomic nucleus can be modified, although this can require very high energies because of the strong force. Nuclear fusion occurs when multiple atomic particles join to form a heavier nucleus, such as through the energetic collision of two nuclei. For example, at the core of the Sun protons require energies of 3 to 10 keV to overcome their mutual repulsion—the coulomb barrier—and fuse together into a single nucleus. Nuclear fission is the opposite process, causing a nucleus to split into two smaller nuclei—usually through radioactive decay. The nucleus can also be modified through bombardment by high energy subatomic particles or photons. If this modifies the number of protons in a nucleus, the atom changes to a different chemical element.
If the mass of the nucleus following a fusion reaction is less than the sum of the masses of the separate particles, then the difference between these two values can be emitted as a type of usable energy (such as a gamma ray, or the kinetic energy of a beta particle), as described by
 The electrons in an atom are attracted to the protons in the nucleus by the
The electrons in an atom are attracted to the protons in the nucleus by the  Each atomic orbital corresponds to a particular energy level of the electron. The electron can change its state to a higher energy level by absorbing a photon with sufficient energy to boost it into the new quantum state. Likewise, through spontaneous emission, an electron in a higher energy state can drop to a lower energy state while radiating the excess energy as a photon. These characteristic energy values, defined by the differences in the energies of the quantum states, are responsible for atomic spectral lines.
The amount of energy needed to remove or add an electron—the electron binding energy—is far less than the binding energy, binding energy of nucleons. For example, it requires only 13.6 eV to strip a Stationary state, ground-state electron from a hydrogen atom, compared to 2.23 ''million'' eV for splitting a deuterium nucleus. Atoms are electric charge, electrically neutral if they have an equal number of protons and electrons. Atoms that have either a deficit or a surplus of electrons are called
Each atomic orbital corresponds to a particular energy level of the electron. The electron can change its state to a higher energy level by absorbing a photon with sufficient energy to boost it into the new quantum state. Likewise, through spontaneous emission, an electron in a higher energy state can drop to a lower energy state while radiating the excess energy as a photon. These characteristic energy values, defined by the differences in the energies of the quantum states, are responsible for atomic spectral lines.
The amount of energy needed to remove or add an electron—the electron binding energy—is far less than the binding energy, binding energy of nucleons. For example, it requires only 13.6 eV to strip a Stationary state, ground-state electron from a hydrogen atom, compared to 2.23 ''million'' eV for splitting a deuterium nucleus. Atoms are electric charge, electrically neutral if they have an equal number of protons and electrons. Atoms that have either a deficit or a surplus of electrons are called
Interactive Chart of Nuclides
] . For 80 of the chemical elements, at least one
 Every element has one or more isotopes that have unstable nuclei that are subject to radioactive decay, causing the nucleus to emit particles or electromagnetic radiation. Radioactivity can occur when the radius of a nucleus is large compared with the radius of the strong force, which only acts over distances on the order of 1 fm.
The most common forms of radioactive decay are:
* Alpha decay: this process is caused when the nucleus emits an alpha particle, which is a helium nucleus consisting of two protons and two neutrons. The result of the emission is a new element with a lower
Every element has one or more isotopes that have unstable nuclei that are subject to radioactive decay, causing the nucleus to emit particles or electromagnetic radiation. Radioactivity can occur when the radius of a nucleus is large compared with the radius of the strong force, which only acts over distances on the order of 1 fm.
The most common forms of radioactive decay are:
* Alpha decay: this process is caused when the nucleus emits an alpha particle, which is a helium nucleus consisting of two protons and two neutrons. The result of the emission is a new element with a lower
 The potential energy of an electron in an atom is negative number, negative relative to when the distance from the nucleus limit at infinity, goes to infinity; its dependence on the electron's position (vector), position reaches the minimum inside the nucleus, roughly in inverse proportion to the distance. In the quantum-mechanical model, a bound electron can occupy only a set of quantum state, states centered on the nucleus, and each state corresponds to a specific energy level; see time-independent Schrödinger equation for a theoretical explanation. An energy level can be measured by the ionization potential, amount of energy needed to unbind the electron from the atom, and is usually given in units of electronvolts (eV). The lowest energy state of a bound electron is called the ground state, i.e. stationary state, while an electron transition to a higher level results in an excited state. The electron's energy increases along with principal quantum number, ''n'' because the (average) distance to the nucleus increases. Dependence of the energy on azimuthal quantum number, is caused not by the electrostatic potential of the nucleus, but by interaction between electrons.
For an electron to atomic electron transition, transition between two different states, e.g. ground state to first excited state, it must absorb or emit a photon at an energy matching the difference in the potential energy of those levels, according to the
The potential energy of an electron in an atom is negative number, negative relative to when the distance from the nucleus limit at infinity, goes to infinity; its dependence on the electron's position (vector), position reaches the minimum inside the nucleus, roughly in inverse proportion to the distance. In the quantum-mechanical model, a bound electron can occupy only a set of quantum state, states centered on the nucleus, and each state corresponds to a specific energy level; see time-independent Schrödinger equation for a theoretical explanation. An energy level can be measured by the ionization potential, amount of energy needed to unbind the electron from the atom, and is usually given in units of electronvolts (eV). The lowest energy state of a bound electron is called the ground state, i.e. stationary state, while an electron transition to a higher level results in an excited state. The electron's energy increases along with principal quantum number, ''n'' because the (average) distance to the nucleus increases. Dependence of the energy on azimuthal quantum number, is caused not by the electrostatic potential of the nucleus, but by interaction between electrons.
For an electron to atomic electron transition, transition between two different states, e.g. ground state to first excited state, it must absorb or emit a photon at an energy matching the difference in the potential energy of those levels, according to the  When a continuous electromagnetic spectrum, spectrum of energy is passed through a gas or plasma, some of the photons are absorbed by atoms, causing electrons to change their energy level. Those excited electrons that remain bound to their atom spontaneously emit this energy as a photon, traveling in a random direction, and so drop back to lower energy levels. Thus the atoms behave like a filter that forms a series of dark absorption bands in the energy output. (An observer viewing the atoms from a view that does not include the continuous spectrum in the background, instead sees a series of emission lines from the photons emitted by the atoms.) Spectroscopy, Spectroscopic measurements of the strength and width of atomic spectral lines allow the composition and physical properties of a substance to be determined.
Close examination of the spectral lines reveals that some display a fine structure splitting. This occurs because of spin–orbit interaction, spin–orbit coupling, which is an interaction between the spin and motion of the outermost electron. When an atom is in an external magnetic field, spectral lines become split into three or more components; a phenomenon called the Zeeman effect. This is caused by the interaction of the magnetic field with the magnetic moment of the atom and its electrons. Some atoms can have multiple electron configurations with the same energy level, which thus appear as a single spectral line. The interaction of the magnetic field with the atom shifts these electron configurations to slightly different energy levels, resulting in multiple spectral lines. The presence of an external electric field can cause a comparable splitting and shifting of spectral lines by modifying the electron energy levels, a phenomenon called the Stark effect.
If a bound electron is in an excited state, an interacting photon with the proper energy can cause stimulated emission of a photon with a matching energy level. For this to occur, the electron must drop to a lower energy state that has an energy difference matching the energy of the interacting photon. The emitted photon and the interacting photon then move off in parallel and with matching phases. That is, the wave patterns of the two photons are synchronized. This physical property is used to make lasers, which can emit a coherent beam of light energy in a narrow frequency band.
When a continuous electromagnetic spectrum, spectrum of energy is passed through a gas or plasma, some of the photons are absorbed by atoms, causing electrons to change their energy level. Those excited electrons that remain bound to their atom spontaneously emit this energy as a photon, traveling in a random direction, and so drop back to lower energy levels. Thus the atoms behave like a filter that forms a series of dark absorption bands in the energy output. (An observer viewing the atoms from a view that does not include the continuous spectrum in the background, instead sees a series of emission lines from the photons emitted by the atoms.) Spectroscopy, Spectroscopic measurements of the strength and width of atomic spectral lines allow the composition and physical properties of a substance to be determined.
Close examination of the spectral lines reveals that some display a fine structure splitting. This occurs because of spin–orbit interaction, spin–orbit coupling, which is an interaction between the spin and motion of the outermost electron. When an atom is in an external magnetic field, spectral lines become split into three or more components; a phenomenon called the Zeeman effect. This is caused by the interaction of the magnetic field with the magnetic moment of the atom and its electrons. Some atoms can have multiple electron configurations with the same energy level, which thus appear as a single spectral line. The interaction of the magnetic field with the atom shifts these electron configurations to slightly different energy levels, resulting in multiple spectral lines. The presence of an external electric field can cause a comparable splitting and shifting of spectral lines by modifying the electron energy levels, a phenomenon called the Stark effect.
If a bound electron is in an excited state, an interacting photon with the proper energy can cause stimulated emission of a photon with a matching energy level. For this to occur, the electron must drop to a lower energy state that has an energy difference matching the energy of the interacting photon. The emitted photon and the interacting photon then move off in parallel and with matching phases. That is, the wave patterns of the two photons are synchronized. This physical property is used to make lasers, which can emit a coherent beam of light energy in a narrow frequency band.
 Quantities of atoms are found in different states of matter that depend on the physical conditions, such as temperature and pressure. By varying the conditions, materials can transition between solids, liquids, gases and plasma (physics), plasmas. Within a state, a material can also exist in different allotropes. An example of this is solid carbon, which can exist as graphite or diamond. Gaseous allotropes exist as well, such as dioxygen and ozone.
At temperatures close to absolute zero, atoms can form a Bose–Einstein condensate, at which point quantum mechanical effects, which are normally only observed at the atomic scale, become apparent on a macroscopic scale. This super-cooled collection of atoms
then behaves as a single super atom, which may allow fundamental checks of quantum mechanical behavior.
Quantities of atoms are found in different states of matter that depend on the physical conditions, such as temperature and pressure. By varying the conditions, materials can transition between solids, liquids, gases and plasma (physics), plasmas. Within a state, a material can also exist in different allotropes. An example of this is solid carbon, which can exist as graphite or diamond. Gaseous allotropes exist as well, such as dioxygen and ozone.
At temperatures close to absolute zero, atoms can form a Bose–Einstein condensate, at which point quantum mechanical effects, which are normally only observed at the atomic scale, become apparent on a macroscopic scale. This super-cooled collection of atoms
then behaves as a single super atom, which may allow fundamental checks of quantum mechanical behavior.
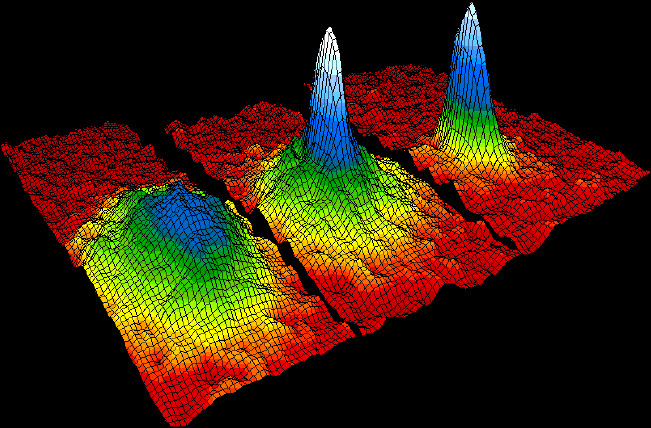
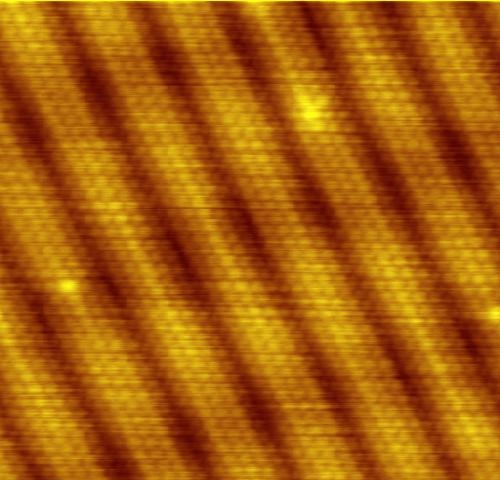 While atoms are too small to be seen, devices such as the scanning tunneling microscope (STM) enable their visualization at the surfaces of solids. The microscope uses the quantum tunneling phenomenon, which allows particles to pass through a barrier that would be insurmountable in the classical perspective. Electrons tunnel through the vacuum between two Biasing, biased electrodes, providing a tunneling current that is exponentially dependent on their separation. One electrode is a sharp tip ideally ending with a single atom. At each point of the scan of the surface the tip's height is adjusted so as to keep the tunneling current at a set value. How much the tip moves to and away from the surface is interpreted as the height profile. For low bias, the microscope images the averaged electron orbitals across closely packed energy levels—the local density of states, density of the electronic states near the Fermi level. Because of the distances involved, both electrodes need to be extremely stable; only then periodicities can be observed that correspond to individual atoms. The method alone is not chemically specific, and cannot identify the atomic species present at the surface.
Atoms can be easily identified by their mass. If an atom is
While atoms are too small to be seen, devices such as the scanning tunneling microscope (STM) enable their visualization at the surfaces of solids. The microscope uses the quantum tunneling phenomenon, which allows particles to pass through a barrier that would be insurmountable in the classical perspective. Electrons tunnel through the vacuum between two Biasing, biased electrodes, providing a tunneling current that is exponentially dependent on their separation. One electrode is a sharp tip ideally ending with a single atom. At each point of the scan of the surface the tip's height is adjusted so as to keep the tunneling current at a set value. How much the tip moves to and away from the surface is interpreted as the height profile. For low bias, the microscope images the averaged electron orbitals across closely packed energy levels—the local density of states, density of the electronic states near the Fermi level. Because of the distances involved, both electrodes need to be extremely stable; only then periodicities can be observed that correspond to individual atoms. The method alone is not chemically specific, and cannot identify the atomic species present at the surface.
Atoms can be easily identified by their mass. If an atom is
 Electrons are thought to exist in the Universe since early stages of the Big Bang. Atomic nuclei forms in nucleosynthesis reactions. In about three minutes Big Bang nucleosynthesis produced most of the helium, lithium, and deuterium in the Universe, and perhaps some of the beryllium and boron.
Ubiquitousness and stability of atoms relies on their binding energy, which means that an atom has a lower energy than an unbound system of the nucleus and electrons. Where the temperature is much higher than ionization potential, the matter exists in the form of plasma (physics), plasma—a gas of positively charged ions (possibly, bare nuclei) and electrons. When the temperature drops below the ionization potential, atoms become statistical physics, statistically favorable. Atoms (complete with bound electrons) became to dominate over electric charge, charged particles 380,000 years after the Big Bang—an epoch called recombination (cosmology), recombination, when the expanding Universe cooled enough to allow electrons to become attached to nuclei.
Since the Big Bang, which produced no carbon or atomic number, heavier elements, atomic nuclei have been combined in stars through the process of nuclear fusion to produce more of the element helium, and (via the triple alpha process) the sequence of elements from carbon up to iron; see stellar nucleosynthesis for details.
Isotopes such as lithium-6, as well as some beryllium and boron are generated in space through cosmic ray spallation. This occurs when a high-energy proton strikes an atomic nucleus, causing large numbers of nucleons to be ejected.
Elements heavier than iron were produced in supernovae and colliding neutron stars through the r-process, and in Asymptotic giant branch, AGB stars through the s-process, both of which involve the capture of neutrons by atomic nuclei. Elements such as lead formed largely through the radioactive decay of heavier elements.
Electrons are thought to exist in the Universe since early stages of the Big Bang. Atomic nuclei forms in nucleosynthesis reactions. In about three minutes Big Bang nucleosynthesis produced most of the helium, lithium, and deuterium in the Universe, and perhaps some of the beryllium and boron.
Ubiquitousness and stability of atoms relies on their binding energy, which means that an atom has a lower energy than an unbound system of the nucleus and electrons. Where the temperature is much higher than ionization potential, the matter exists in the form of plasma (physics), plasma—a gas of positively charged ions (possibly, bare nuclei) and electrons. When the temperature drops below the ionization potential, atoms become statistical physics, statistically favorable. Atoms (complete with bound electrons) became to dominate over electric charge, charged particles 380,000 years after the Big Bang—an epoch called recombination (cosmology), recombination, when the expanding Universe cooled enough to allow electrons to become attached to nuclei.
Since the Big Bang, which produced no carbon or atomic number, heavier elements, atomic nuclei have been combined in stars through the process of nuclear fusion to produce more of the element helium, and (via the triple alpha process) the sequence of elements from carbon up to iron; see stellar nucleosynthesis for details.
Isotopes such as lithium-6, as well as some beryllium and boron are generated in space through cosmic ray spallation. This occurs when a high-energy proton strikes an atomic nucleus, causing large numbers of nucleons to be ejected.
Elements heavier than iron were produced in supernovae and colliding neutron stars through the r-process, and in Asymptotic giant branch, AGB stars through the s-process, both of which involve the capture of neutrons by atomic nuclei. Elements such as lead formed largely through the radioactive decay of heavier elements.
nucleus
Nucleus ( : nuclei) is a Latin word for the seed inside a fruit. It most often refers to:
*Atomic nucleus, the very dense central region of an atom
* Cell nucleus, a central organelle of a eukaryotic cell, containing most of the cell's DNA
Nucl ...
of protons and neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons behav ...
s surrounded by a cloud of electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary partic ...
s. The atom is the basic particle of the chemical element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler sub ...
s, and the chemical elements are distinguished from each other by the number of protons that are in their atoms. For example, any atom that contains 11 protons is sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable ...
, and any atom that contains 29 protons is copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish ...
. The number of neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons behav ...
s defines the isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers ( mass number ...
of the element.
Atoms are extremely small, typically around 100 picometer
The picometre (international spelling as used by the International Bureau of Weights and Measures; SI symbol: pm) or picometer ( American spelling) is a unit of length in the International System of Units (SI), equal to , or one trillionth ...
s across. A human hair is about a million carbon atoms wide. This is smaller than the shortest wavelength of visible light, which means humans cannot see atoms with conventional microscopes. Atoms are so small that accurately predicting their behavior using classical physics is not possible due to quantum effects
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, ...
.
More than 99.94% of an atom's mass
Mass is an intrinsic property of a body. It was traditionally believed to be related to the quantity of matter in a physical body, until the discovery of the atom and particle physics. It was found that different atoms and different element ...
is in the nucleus. The protons have a positive electric charge
Electric charge is the physical property of matter that causes charged matter to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative'' (commonly carried by protons and electrons respecti ...
, the electrons have a negative electric charge, and the neutrons have no electric charge. If the number of protons and electrons are equal, then the atom is electrically neutral. If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively – such atoms are called ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
s.
The electrons of an atom are attracted to the protons in an atomic nucleus by the electromagnetic force
In physics, electromagnetism is an interaction that occurs between particles with electric charge. It is the second-strongest of the four fundamental interactions, after the strong force, and it is the dominant force in the interactions o ...
. The protons and neutrons in the nucleus are attracted to each other by the nuclear force
The nuclear force (or nucleon–nucleon interaction, residual strong force, or, historically, strong nuclear force) is a force that acts between the protons and neutrons of atoms. Neutrons and protons, both nucleons, are affected by the nucl ...
. This force is usually stronger than the electromagnetic force that repels the positively charged protons from one another. Under certain circumstances, the repelling electromagnetic force becomes stronger than the nuclear force. In this case, the nucleus splits
A split (commonly referred to as splits or the splits) is a physical position in which the legs are in line with each other and extended in opposite directions. Splits are commonly performed in various athletic activities, including dance, fig ...
and leaves behind different elements. This is a form of nuclear decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive Decay chain, disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nucl ...
.
Atoms can attach to one or more other atoms by chemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing o ...
s to form chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one ele ...
s such as molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bio ...
s or crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macr ...
s. The ability of atoms to attach and detach is responsible for most of the physical changes observed in nature. Chemistry is the discipline that studies these changes.
History of atomic theory
In philosophy
The basic idea that matter is made up of tiny indivisible particles is an old idea that appeared in many ancient cultures. The word ''atom'' is derived from theancient Greek
Ancient Greek includes the forms of the Greek language used in ancient Greece and the ancient world from around 1500 BC to 300 BC. It is often roughly divided into the following periods: Mycenaean Greek (), Dark Ages (), the Archaic p ...
word ''atomos'', which means "uncuttable". This ancient idea was based in philosophical reasoning rather than scientific reasoning. Modern atomic theory is not based on these old concepts. In the early 19th century, the scientist John Dalton
John Dalton (; 5 or 6 September 1766 – 27 July 1844) was an English chemist, physicist and meteorologist. He is best known for introducing the atomic theory into chemistry, and for his research into colour blindness, which he had. Colour b ...
noticed that chemical elements seemed to combine with each other by discrete units of weight, and he decided to use the word "atom" to refer to these units, as he thought these were the fundamental units of matter. About a century later it was discovered that Dalton's atoms are not actually indivisible, but the term stuck.
Dalton's law of multiple proportions
 In the early 1800s, the English chemist
In the early 1800s, the English chemist John Dalton
John Dalton (; 5 or 6 September 1766 – 27 July 1844) was an English chemist, physicist and meteorologist. He is best known for introducing the atomic theory into chemistry, and for his research into colour blindness, which he had. Colour b ...
compiled experimental data gathered by himself and other scientists and discovered a pattern now known as the "law of multiple proportions
In chemistry, the law of multiple proportions states that if two elements form more than one compound, then the ratios of the masses of the second element which combine with a fixed mass of the first element will always be ratios of small whole ...
". He noticed that in chemical compounds which contain a particular chemical element, the content of that element in these compounds will differ in weight by ratios of small whole numbers. This pattern suggested that each chemical element combines with other elements by a basic unit of weight, and Dalton decided to call these units "atoms".
For example, there are two types of tin oxide
Tin is a chemical element with the symbol Sn (from la, stannum) and atomic number 50. Tin is a silvery-coloured metal.
Tin is soft enough to be cut with little force and a bar of tin can be bent by hand with little effort. When bent, t ...
: one is a grey powder that is 88.1% tin and 11.9% oxygen, and the other is a white powder that is 78.7% tin and 21.3% oxygen. Adjusting these figures, in the grey powder there is about 13.5 g of oxygen for every 100 g of tin, and in the white powder there is about 27 g of oxygen for every 100 g of tin. 13.5 and 27 form a ratio of 1:2. Dalton concluded that in these oxides, for every tin atom there are one or two oxygen atoms respectively (SnO
Tin(II) oxide (stannous oxide) is a compound with the formula SnO. It is composed of tin and oxygen where tin has the oxidation state of +2. There are two forms, a stable blue-black form and a metastable red form.
Preparation and reactions
Blue ...
and SnO2).
Dalton also analyzed iron oxide
Iron oxides are chemical compounds composed of iron and oxygen. Several iron oxides are recognized. All are black magnetic solids. Often they are non-stoichiometric. Oxyhydroxides are a related class of compounds, perhaps the best known of w ...
s. There is one type of iron oxide that is a black powder which is 78.1% iron and 21.9% oxygen; and there is another iron oxide that is a red powder which is 70.4% iron and 29.6% oxygen. Adjusting these figures, in the black powder there is about 28 g of oxygen for every 100 g of iron, and in the red powder there is about 42 g of oxygen for every 100 g of iron. 28 and 42 form a ratio of 2:3. Dalton concluded that in these oxides, for every two atoms of iron, there are two or three atoms of oxygen respectively ( Fe2O2 and Fe2O3).
As a final example: nitrous oxide
Nitrous oxide (dinitrogen oxide or dinitrogen monoxide), commonly known as laughing gas, nitrous, or nos, is a chemical compound, an oxide of nitrogen with the formula . At room temperature, it is a colourless non-flammable gas, and has ...
is 63.3% nitrogen and 36.7% oxygen, nitric oxide
Nitric oxide (nitrogen oxide or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its ...
is 44.05% nitrogen and 55.95% oxygen, and nitrogen dioxide
Nitrogen dioxide is a chemical compound with the formula . It is one of several nitrogen oxides. is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year for use primarily in the productio ...
is 29.5% nitrogen and 70.5% oxygen. Adjusting these figures, in nitrous oxide there is 80 g of oxygen for every 140 g of nitrogen, in nitric oxide there is about 160 g of oxygen for every 140 g of nitrogen, and in nitrogen dioxide there is 320 g of oxygen for every 140 g of nitrogen. 80, 160, and 320 form a ratio of 1:2:4. The respective formulas for these oxides are N2O, NO, and NO2.
Kinetic theory of gases
In 1738Daniel Bernoulli
Daniel Bernoulli FRS (; – 27 March 1782) was a Swiss mathematician and physicist and was one of the many prominent mathematicians in the Bernoulli family from Basel. He is particularly remembered for his applications of mathematics to mech ...
and a number of other scientists found that they could better explain the behavior of gases by describing them as collections of sub-microscopic particles and modelling their behavior using statistics and probability
Probability is the branch of mathematics concerning numerical descriptions of how likely an Event (probability theory), event is to occur, or how likely it is that a proposition is true. The probability of an event is a number between 0 and ...
. Unlike Dalton's atomic theory, the kinetic theory of gases describes not how gases react chemically with each other to form compounds, but how they behave physically: diffusion, viscosity, conductivity, pressure, etc.
Brownian motion
In 1827, botanist Robert Brown used a microscope to look at dust grains floating in water and discovered that they moved about erratically, a phenomenon that became known as "Brownian motion
Brownian motion, or pedesis (from grc, πήδησις "leaping"), is the random motion of particles suspended in a medium (a liquid or a gas).
This pattern of motion typically consists of random fluctuations in a particle's position insi ...
". This was thought to be caused by water molecules knocking the grains about. In 1905, Albert Einstein
Albert Einstein ( ; ; 14 March 1879 – 18 April 1955) was a German-born theoretical physicist, widely acknowledged to be one of the greatest and most influential physicists of all time. Einstein is best known for developing the theor ...
proved the reality of these molecules and their motions by producing the first statistical physics
Statistical physics is a branch of physics that evolved from a foundation of statistical mechanics, which uses methods of probability theory and statistics, and particularly the mathematical tools for dealing with large populations and approxi ...
analysis of Brownian motion
Brownian motion, or pedesis (from grc, πήδησις "leaping"), is the random motion of particles suspended in a medium (a liquid or a gas).
This pattern of motion typically consists of random fluctuations in a particle's position insi ...
. French physicist Jean Perrin
Jean Baptiste Perrin (30 September 1870 – 17 April 1942) was a French physicist who, in his studies of the Brownian motion of minute particles suspended in liquids (sedimentation equilibrium), verified Albert Einstein’s explanation of this ...
used Einstein's work to experimentally determine the mass and dimensions of molecules, thereby providing physical evidence for the particle nature of matter.
Discovery of the electron
J. J. Thomson
Sir Joseph John Thomson (18 December 1856 – 30 August 1940) was a British physicist and Nobel Laureate in Physics, credited with the discovery of the electron, the first subatomic particle to be discovered.
In 1897, Thomson showed that ...
discovered that cathode ray
Cathode rays or electron beam (e-beam) are streams of electrons observed in discharge tubes. If an evacuated glass tube is equipped with two electrodes and a voltage is applied, glass behind the positive electrode is observed to glow, due to el ...
s are not electromagnetic waves but made of particles because they can be deflected by electrical and magnetic fields. He measured these particles to be 1,800 times lighter than hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
(the lightest atom). Thomson concluded that these particles came from the atoms within the cathode — they were ''subatomic'' particles. He called these new particles ''corpuscles'' but they were later renamed ''electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary partic ...
s''. Thomson also showed that electrons were identical to particles given off by photoelectric
The photoelectric effect is the emission of electrons when electromagnetic radiation, such as light, hits a material. Electrons emitted in this manner are called photoelectrons. The phenomenon is studied in condensed matter physics, and solid sta ...
and radioactive materials. It was quickly recognized that electrons are the particles that carry electric current
An electric current is a stream of charged particles, such as electrons or ions, moving through an electrical conductor or space. It is measured as the net rate of flow of electric charge through a surface or into a control volume. The movin ...
s in metal wires. Thomson concluded that these electrons emerged from the very atoms of the cathode in his instruments, which meant that atoms are not indivisible as Dalton thought.
Discovery of the nucleus
J. J. Thomson
Sir Joseph John Thomson (18 December 1856 – 30 August 1940) was a British physicist and Nobel Laureate in Physics, credited with the discovery of the electron, the first subatomic particle to be discovered.
In 1897, Thomson showed that ...
thought that the negatively-charged electrons were distributed throughout the atom in a sea of positive charge that was distributed across the whole volume of the atom. This model is sometimes known as the plum pudding model
The plum pudding model is one of several historical scientific models of the atom. First proposed by J. J. Thomson in 1904 soon after the discovery of the electron, but before the discovery of the atomic nucleus, the model tried to explain two ...
.
Ernest Rutherford
Ernest Rutherford, 1st Baron Rutherford of Nelson, (30 August 1871 – 19 October 1937) was a New Zealand physicist who came to be known as the father of nuclear physics.
''Encyclopædia Britannica'' considers him to be the greatest ...
and his colleagues Hans Geiger
Johannes Wilhelm "Hans" Geiger (; ; 30 September 1882 – 24 September 1945) was a German physicist. He is best known as the co-inventor of the detector component of the Geiger counter and for the Geiger–Marsden experiment which discover ...
and Ernest Marsden
Sir Ernest Marsden (19 February 1889 – 15 December 1970) was an English-New Zealand physicist. He is recognised internationally for his contributions to science while working under Ernest Rutherford, which led to the discovery of new theorie ...
came to doubt the Thomson model after they encountered difficulties when they tried to build an instrument to measure the charge-to-mass ratio of alpha particles
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be produce ...
(these are positively-charged particles emitted by certain radioactive substances such as radium
Radium is a chemical element with the symbol Ra and atomic number 88. It is the sixth element in group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is silvery-white, but it readily reacts with nitrogen (rathe ...
). The alpha particles were being scattered by the air in the detection chamber, which made the measurements unreliable. Thomson had encountered a similar problem in his work on cathode rays, which he solved by creating a near-perfect vacuum in his instruments. Rutherford didn't think he'd run into this same problem because alpha particles are much heavier than electrons. According to Thomson's model of the atom, the positive charge in the atom is not concentrated enough to produce an electric field strong enough to deflect an alpha particle, and the electrons are so lightweight they should be pushed aside effortlessly by the much heavier alpha particles. Yet there was scattering, so Rutherford and his colleagues decided to investigate this scattering carefully. Heilbron (2003). ''Ernest Rutherford and the Explosion of Atoms'', pp. 64-68
Between 1908 and 1913, Rutherford and his colleagues performed a series of experiments in which they bombarded thin foils of metal with alpha particles. They spotted alpha particles being deflected by angles greater than 90°. To explain this, Rutherford proposed that the positive charge of the atom is not distributed throughout the atom's volume as Thomson believed, but is concentrated in a tiny nucleus at the center. Only such an intense concentration of charge could produce an electric field strong enough to deflect the alpha particles as observed.
Discovery of isotopes
While experimenting with the products ofradioactive decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consid ...
, in 1913 radiochemist Frederick Soddy
Frederick Soddy FRS (2 September 1877 – 22 September 1956) was an English radiochemist who explained, with Ernest Rutherford, that radioactivity is due to the transmutation of elements, now known to involve nuclear reactions. He also pro ...
discovered that there appeared to be more than one type of atom at each position on the periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ...
. The term isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers ( mass number ...
was coined by Margaret Todd as a suitable name for different atoms that belong to the same element. J. J. Thomson created a technique for isotope separation
Isotope separation is the process of concentrating specific isotopes of a chemical element by removing other isotopes. The use of the nuclides produced is varied. The largest variety is used in research (e.g. in chemistry where atoms of "marker" ...
through his work on es, which subsequently led to the discovery of stable isotope
The term stable isotope has a meaning similar to stable nuclide, but is preferably used when speaking of nuclides of a specific element. Hence, the plural form stable isotopes usually refers to isotopes of the same element. The relative abundanc ...
s.
Bohr model
 In 1913, the physicist
In 1913, the physicist Niels Bohr
Niels Henrik David Bohr (; 7 October 1885 – 18 November 1962) was a Danish physicist who made foundational contributions to understanding atomic structure and quantum theory, for which he received the Nobel Prize in Physics in 1922 ...
proposed a model in which the electrons of an atom were assumed to orbit the nucleus but could only do so in a finite set of orbits, and could jump between these orbits only in discrete changes of energy corresponding to absorption or radiation of a photon. This quantization was used to explain why the electrons' orbits are stable (given that normally, charges in acceleration, including circular motion, lose kinetic energy which is emitted as electromagnetic radiation, see ''synchrotron radiation
Synchrotron radiation (also known as magnetobremsstrahlung radiation) is the electromagnetic radiation emitted when relativistic charged particles are subject to an acceleration perpendicular to their velocity (). It is produced artificially in ...
'') and why elements absorb and emit electromagnetic radiation in discrete spectra.
Later in the same year Henry Moseley
Henry Gwyn Jeffreys Moseley (; 23 November 1887 – 10 August 1915) was an English physicist, whose contribution to the science of physics was the justification from physical laws of the previous empirical and chemical concept of the atomic num ...
provided additional experimental evidence in favor of Niels Bohr's theory. These results refined Ernest Rutherford
Ernest Rutherford, 1st Baron Rutherford of Nelson, (30 August 1871 – 19 October 1937) was a New Zealand physicist who came to be known as the father of nuclear physics.
''Encyclopædia Britannica'' considers him to be the greatest ...
's and Antonius van den Broek
Antonius Johannes van den Broek (4 May 1870, Zoetermeer – 25 October 1926, Bilthoven) was a Dutch amateur physicist notable for being the first who realized that the number of an element in the periodic table (now called atomic number) correspond ...
's model, which proposed that the atom contains in its nucleus
Nucleus ( : nuclei) is a Latin word for the seed inside a fruit. It most often refers to:
*Atomic nucleus, the very dense central region of an atom
* Cell nucleus, a central organelle of a eukaryotic cell, containing most of the cell's DNA
Nucl ...
a number of positive nuclear charge In atomic physics, the effective nuclear charge is the actual amount of positive (nuclear) charge experienced by an electron in a multi-electron atom. The term "effective" is used because the shielding effect of negatively charged electrons prevent ...
s that is equal to its (atomic) number in the periodic table. Until these experiments, atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every ...
was not known to be a physical and experimental quantity. That it is equal to the atomic nuclear charge remains the accepted atomic model today.
Chemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing o ...
s between atoms were explained by Gilbert Newton Lewis
Gilbert Newton Lewis (October 23 or October 25, 1875 – March 23, 1946) was an American physical chemist and a Dean of the College of Chemistry at University of California, Berkeley. Lewis was best known for his discovery of the covalent bond ...
in 1916, as the interactions between their constituent electrons. As the chemical properties
A chemical property is any of a material's properties that becomes evident during, or after, a chemical reaction; that is, any quality that can be established only by changing a substance's chemical identity.William L. Masterton, Cecile N. Hurley, ...
of the elements were known to largely repeat themselves according to the periodic law
Periodic trends are specific patterns that are present in the periodic table that illustrate different aspects of a certain element. They were discovered by the Russian chemist Dmitri Mendeleev in the year 1863. Major periodic trends include atom ...
, in 1919 the American chemist Irving Langmuir suggested that this could be explained if the electrons in an atom were connected or clustered in some manner. Groups of electrons were thought to occupy a set of electron shell
In chemistry and atomic physics, an electron shell may be thought of as an orbit followed by electrons around an atom's Atomic nucleus, nucleus. The closest shell to the nucleus is called the "1 shell" (also called the "K shell"), followed by t ...
s about the nucleus.
The Bohr model of the atom was the first complete physical model of the atom. It described the overall structure of the atom, how atoms bond to each other, and predicted the spectral lines of hydrogen. Bohr's model was not perfect and was soon superseded by the more accurate Schrödinger model, but it was sufficient to evaporate any remaining doubts that matter is composed of atoms. For chemists, the idea of the atom had been a useful heuristic tool, but physicists had doubts as to whether matter really is made up of atoms as nobody had yet developed a complete physical model of the atom.
The Schrödinger model
TheStern–Gerlach experiment
The Stern–Gerlach experiment demonstrated that the spatial orientation of angular momentum is quantized. Thus an atomic-scale system was shown to have intrinsically quantum properties. In the original experiment, silver atoms were sent throug ...
of 1922 provided further evidence of the quantum nature of atomic properties. When a beam of silver atoms was passed through a specially shaped magnetic field, the beam was split in a way correlated with the direction of an atom's angular momentum, or spin
Spin or spinning most often refers to:
* Spinning (textiles), the creation of yarn or thread by twisting fibers together, traditionally by hand spinning
* Spin, the rotation of an object around a central axis
* Spin (propaganda), an intentionally b ...
. As this spin direction is initially random, the beam would be expected to deflect in a random direction. Instead, the beam was split into two directional components, corresponding to the atomic spin
Spin or spinning most often refers to:
* Spinning (textiles), the creation of yarn or thread by twisting fibers together, traditionally by hand spinning
* Spin, the rotation of an object around a central axis
* Spin (propaganda), an intentionally b ...
being oriented up or down with respect to the magnetic field.
In 1925, Werner Heisenberg
Werner Karl Heisenberg () (5 December 1901 – 1 February 1976) was a German theoretical physicist and one of the main pioneers of the theory of quantum mechanics. He published his work in 1925 in a breakthrough paper. In the subsequent series ...
published the first consistent mathematical formulation of quantum mechanics (matrix mechanics
Matrix mechanics is a formulation of quantum mechanics created by Werner Heisenberg, Max Born, and Pascual Jordan in 1925. It was the first conceptually autonomous and logically consistent formulation of quantum mechanics. Its account of quantum ...
). One year earlier, Louis de Broglie
Louis Victor Pierre Raymond, 7th Duc de Broglie (, also , or ; 15 August 1892 – 19 March 1987) was a French physicist and aristocrat who made groundbreaking contributions to quantum theory. In his 1924 PhD thesis, he postulated the wave n ...
had proposed the de Broglie hypothesis
Matter waves are a central part of the theory of quantum mechanics, being an example of wave–particle duality. All matter exhibits wave-like behavior. For example, a beam of electrons can be diffraction, diffracted just like a beam of light or ...
: that all particles behave like waves to some extent, and in 1926 Erwin Schrödinger
Erwin Rudolf Josef Alexander Schrödinger (, ; ; 12 August 1887 – 4 January 1961), sometimes written as or , was a Nobel Prize-winning Austrian physicist with Irish citizenship who developed a number of fundamental results in quantum theo ...
used this idea to develop the Schrödinger equation
The Schrödinger equation is a linear partial differential equation that governs the wave function of a quantum-mechanical system. It is a key result in quantum mechanics, and its discovery was a significant landmark in the development of th ...
, a mathematical model of the atom (wave mechanics) that described the electrons as three-dimensional waveform
In electronics, acoustics, and related fields, the waveform of a signal is the shape of its graph as a function of time, independent of its time and magnitude scales and of any displacement in time.David Crecraft, David Gorham, ''Electron ...
s rather than point particles.
A consequence of using waveforms to describe particles is that it is mathematically impossible to obtain precise values for both the position
Position often refers to:
* Position (geometry), the spatial location (rather than orientation) of an entity
* Position, a job or occupation
Position may also refer to:
Games and recreation
* Position (poker), location relative to the dealer
* ...
and momentum
In Newtonian mechanics, momentum (more specifically linear momentum or translational momentum) is the product of the mass and velocity of an object. It is a vector quantity, possessing a magnitude and a direction. If is an object's mass ...
of a particle at a given point in time. This became known as the uncertainty principle
In quantum mechanics, the uncertainty principle (also known as Heisenberg's uncertainty principle) is any of a variety of mathematical inequalities asserting a fundamental limit to the accuracy with which the values for certain pairs of physic ...
, formulated by Werner Heisenberg
Werner Karl Heisenberg () (5 December 1901 – 1 February 1976) was a German theoretical physicist and one of the main pioneers of the theory of quantum mechanics. He published his work in 1925 in a breakthrough paper. In the subsequent series ...
in 1927. In this concept, for a given accuracy in measuring a position one could only obtain a range of probable values for momentum, and vice versa.
This model was able to explain observations of atomic behavior that previous models could not, such as certain structural and spectral
''Spectral'' is a 2016 3D military science fiction, supernatural horror fantasy and action-adventure thriller war film directed by Nic Mathieu. Written by himself, Ian Fried, and George Nolfi from a story by Fried and Mathieu. The film stars Ja ...
patterns of atoms larger than hydrogen. Thus, the planetary model of the atom was discarded in favor of one that described atomic orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in an ...
zones around the nucleus where a given electron is most likely to be observed.
Discovery of the neutron
The development of themass spectrometer
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a '' mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is u ...
allowed the mass of atoms to be measured with increased accuracy. The device uses a magnet to bend the trajectory of a beam of ions, and the amount of deflection is determined by the ratio of an atom's mass to its charge. The chemist Francis William Aston
Francis William Aston FRS (1 September 1877 – 20 November 1945) was a British chemist and physicist who won the 1922 Nobel Prize in Chemistry for his discovery, by means of his mass spectrograph, of isotopes in many non-radioactive elements a ...
used this instrument to show that isotopes had different masses. The atomic mass
The atomic mass (''m''a or ''m'') is the mass of an atom. Although the SI unit of mass is the kilogram (symbol: kg), atomic mass is often expressed in the non-SI unit dalton (symbol: Da) – equivalently, unified atomic mass unit (u). 1&nbs ...
of these isotopes varied by integer amounts, called the whole number rule
In chemistry, the whole number rule states that the masses of the isotopes are whole number multiples of the mass of the hydrogen atom. The rule is a modified version of Prout's hypothesis proposed in 1815, to the effect that atomic weights are m ...
. The explanation for these different isotopes awaited the discovery of the neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons behav ...
, an uncharged particle with a mass similar to the proton, by the physicist James Chadwick
Sir James Chadwick, (20 October 1891 – 24 July 1974) was an English physicist who was awarded the 1935 Nobel Prize in Physics for his discovery of the neutron in 1932. In 1941, he wrote the final draft of the MAUD Report, which insp ...
in 1932. Isotopes were then explained as elements with the same number of protons, but different numbers of neutrons within the nucleus.
Fission, high-energy physics and condensed matter
In 1938, the German chemistOtto Hahn
Otto Hahn (; 8 March 1879 – 28 July 1968) was a German chemist who was a pioneer in the fields of radioactivity and radiochemistry. He is referred to as the father of nuclear chemistry and father of nuclear fission. Hahn and Lise Meitner ...
, a student of Rutherford, directed neutrons onto uranium atoms expecting to get transuranium element
The transuranium elements (also known as transuranic elements) are the chemical elements with atomic numbers greater than 92, which is the atomic number of uranium. All of these elements are unstable and decay radioactively into other elements. ...
s. Instead, his chemical experiments showed barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element.
...
as a product. A year later, Lise Meitner
Elise Meitner ( , ; 7 November 1878 – 27 October 1968) was an Austrian-Swedish physicist who was one of those responsible for the discovery of the element protactinium and nuclear fission. While working at the Kaiser Wilhelm Institute on ra ...
and her nephew Otto Frisch
Otto Robert Frisch FRS (1 October 1904 – 22 September 1979) was an Austrian-born British physicist who worked on nuclear physics. With Lise Meitner he advanced the first theoretical explanation of nuclear fission (coining the term) and first ...
verified that Hahn's result were the first experimental ''nuclear fission''. In 1944, Hahn received the Nobel Prize in Chemistry
)
, image = Nobel Prize.png
, alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFR•" then "NOBEL", and on the right, the text (smaller) "NAT•" then "M ...
. Despite Hahn's efforts, the contributions of Meitner and Frisch were not recognized.
In the 1950s, the development of improved particle accelerator
A particle accelerator is a machine that uses electromagnetic fields to propel electric charge, charged particles to very high speeds and energies, and to contain them in well-defined particle beam, beams.
Large accelerators are used for fun ...
s and particle detector
In experimental and applied particle physics, nuclear physics, and nuclear engineering, a particle detector, also known as a radiation detector, is a device used to detect, track, and/or identify ionizing particles, such as those produced by ...
s allowed scientists to study the impacts of atoms moving at high energies. Neutrons and protons were found to be hadron
In particle physics, a hadron (; grc, ἁδρός, hadrós; "stout, thick") is a composite subatomic particle made of two or more quarks held together by the strong interaction. They are analogous to molecules that are held together by the ele ...
s, or composites of smaller particles called quark
A quark () is a type of elementary particle and a fundamental constituent of matter. Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nuclei. All common ...
s. The standard model of particle physics
The Standard Model of particle physics is the theory describing three of the four known fundamental forces (electromagnetic, weak and strong interactions - excluding gravity) in the universe and classifying all known elementary particles. It wa ...
was developed that so far has successfully explained the properties of the nucleus in terms of these sub-atomic particles and the forces that govern their interactions.
Structure
Subatomic particles
Though the word ''atom'' originally denoted a particle that cannot be cut into smaller particles, in modern scientific usage the atom is composed of various subatomic particles. The constituent particles of an atom are theelectron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary partic ...
, the proton and the neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons behav ...
.
The electron is by far the least massive of these particles at , with a negative electrical charge
Electricity is the set of physical phenomena associated with the presence and motion of matter that has a property of electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described by ...
and a size that is too small to be measured using available techniques. It was the lightest particle with a positive rest mass measured, until the discovery of neutrino
A neutrino ( ; denoted by the Greek letter ) is a fermion (an elementary particle with spin of ) that interacts only via the weak interaction and gravity. The neutrino is so named because it is electrically neutral and because its rest mass ...
mass. Under ordinary conditions, electrons are bound to the positively charged nucleus by the attraction created from opposite electric charges. If an atom has more or fewer electrons than its atomic number, then it becomes respectively negatively or positively charged as a whole; a charged atom is called an ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
. Electrons have been known since the late 19th century, mostly thanks to J.J. Thomson
Sir Joseph John Thomson (18 December 1856 – 30 August 1940) was a British physicist and Nobel Laureate in Physics, credited with the discovery of the electron, the first subatomic particle to be discovered.
In 1897, Thomson showed that ...
; see history of subatomic physics
The idea that matter consists of smaller particles and that there exists a limited number of sorts of primary, smallest particles in nature has existed in natural philosophy at least since the 6th century BC. Such ideas gained physical credibility ...
for details.
Protons have a positive charge and a mass 1,836 times that of the electron, at . The number of protons in an atom is called its atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every ...
. Ernest Rutherford
Ernest Rutherford, 1st Baron Rutherford of Nelson, (30 August 1871 – 19 October 1937) was a New Zealand physicist who came to be known as the father of nuclear physics.
''Encyclopædia Britannica'' considers him to be the greatest ...
(1919) observed that nitrogen under alpha-particle bombardment ejects what appeared to be hydrogen nuclei. By 1920 he had accepted that the hydrogen nucleus is a distinct particle within the atom and named it proton.
Neutrons have no electrical charge and have a free mass of 1,839 times the mass of the electron, or .Mohr, P.J.; Taylor, B.N. and Newell, D.B. (2014)"The 2014 CODATA Recommended Values of the Fundamental Physical Constants"
(Web Version 7.0). The database was developed by J. Baker, M. Douma, and S. Kotochigova. (2014). National Institute of Standards and Technology, Gaithersburg, Maryland 20899. Neutrons are the heaviest of the three constituent particles, but their mass can be reduced by the
nuclear binding energy
Nuclear binding energy in experimental physics is the minimum energy that is required to disassemble the nucleus of an atom into its constituent protons and neutrons, known collectively as nucleons. The binding energy for stable nuclei is always ...
. Neutrons and protons (collectively known as nucleon
In physics and chemistry, a nucleon is either a proton or a neutron, considered in its role as a component of an atomic nucleus. The number of nucleons in a nucleus defines the atom's mass number (nucleon number).
Until the 1960s, nucleons w ...
s) have comparable dimensions—on the order of —although the 'surface' of these particles is not sharply defined. The neutron was discovered in 1932 by the English physicist James Chadwick
Sir James Chadwick, (20 October 1891 – 24 July 1974) was an English physicist who was awarded the 1935 Nobel Prize in Physics for his discovery of the neutron in 1932. In 1941, he wrote the final draft of the MAUD Report, which insp ...
.
In the Standard Model
The Standard Model of particle physics is the theory describing three of the four known fundamental forces ( electromagnetic, weak and strong interactions - excluding gravity) in the universe and classifying all known elementary particles. I ...
of physics, electrons are truly elementary particles with no internal structure, whereas protons and neutrons are composite particles composed of elementary particle
In particle physics, an elementary particle or fundamental particle is a subatomic particle that is not composed of other particles. Particles currently thought to be elementary include electrons, the fundamental fermions (quarks, leptons, antiq ...
s called quark
A quark () is a type of elementary particle and a fundamental constituent of matter. Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nuclei. All common ...
s. There are two types of quarks in atoms, each having a fractional electric charge. Protons are composed of two up quark
The up quark or u quark (symbol: u) is the lightest of all quarks, a type of elementary particle, and a significant constituent of matter. It, along with the down quark, forms the neutrons (one up quark, two down quarks) and protons (two up qua ...
s (each with charge +) and one down quark
The down quark or d quark (symbol: d) is the second-lightest of all quarks, a type of elementary particle, and a major constituent of matter. Together with the up quark, it forms the neutrons (one up quark, two down quarks) and protons (two u ...
(with a charge of −). Neutrons consist of one up quark and two down quarks. This distinction accounts for the difference in mass and charge between the two particles.
The quarks are held together by the strong interaction
The strong interaction or strong force is a fundamental interaction that confines quarks into proton, neutron, and other hadron particles. The strong interaction also binds neutrons and protons to create atomic nuclei, where it is called th ...
(or strong force), which is mediated by gluon
A gluon ( ) is an elementary particle that acts as the exchange particle (or gauge boson) for the strong force between quarks. It is analogous to the exchange of photons in the electromagnetic force between two charged particles. Gluons bi ...
s. The protons and neutrons, in turn, are held to each other in the nucleus by the nuclear force
The nuclear force (or nucleon–nucleon interaction, residual strong force, or, historically, strong nuclear force) is a force that acts between the protons and neutrons of atoms. Neutrons and protons, both nucleons, are affected by the nucl ...
, which is a residuum of the strong force that has somewhat different range-properties (see the article on the nuclear force for more). The gluon is a member of the family of gauge boson
In particle physics, a gauge boson is a bosonic elementary particle that acts as the force carrier for elementary fermions. Elementary particles, whose interactions are described by a gauge theory, interact with each other by the exchange of gau ...
s, which are elementary particles that mediate physical forces.
Nucleus
atomic nucleus
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden experiments, Geiger–Marsden gold foil experiment. After th ...
, and are collectively called nucleon
In physics and chemistry, a nucleon is either a proton or a neutron, considered in its role as a component of an atomic nucleus. The number of nucleons in a nucleus defines the atom's mass number (nucleon number).
Until the 1960s, nucleons w ...
s. The radius of a nucleus is approximately equal to femtometres, where is the total number of nucleons. This is much smaller than the radius of the atom, which is on the order of 105 fm. The nucleons are bound together by a short-ranged attractive potential called the residual strong force. At distances smaller than 2.5 fm this force is much more powerful than the electrostatic force that causes positively charged protons to repel each other.
Atoms of the same chemical element, element have the same number of protons, called the atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every ...
. Within a single element, the number of neutrons may vary, determining the isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers ( mass number ...
of that element. The total number of protons and neutrons determine the nuclide. The number of neutrons relative to the protons determines the stability of the nucleus, with certain isotopes undergoing radioactive decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consid ...
.
The proton, the electron, and the neutron are classified as fermions. Fermions obey the Pauli exclusion principle which prohibits ''identical particles, identical'' fermions, such as multiple protons, from occupying the same quantum state at the same time. Thus, every proton in the nucleus must occupy a quantum state different from all other protons, and the same applies to all neutrons of the nucleus and to all electrons of the electron cloud.
A nucleus that has a different number of protons than neutrons can potentially drop to a lower energy state through a radioactive decay that causes the number of protons and neutrons to more closely match. As a result, atoms with matching numbers of protons and neutrons are more stable against decay, but with increasing atomic number, the mutual repulsion of the protons requires an increasing proportion of neutrons to maintain the stability of the nucleus.
Albert Einstein
Albert Einstein ( ; ; 14 March 1879 – 18 April 1955) was a German-born theoretical physicist, widely acknowledged to be one of the greatest and most influential physicists of all time. Einstein is best known for developing the theor ...
's mass-energy equivalence formula, , where is the mass loss and is the speed of light. This deficit is part of the binding energy of the new nucleus, and it is the non-recoverable loss of the energy that causes the fused particles to remain together in a state that requires this energy to separate.
The fusion of two nuclei that create larger nuclei with lower atomic numbers than iron and nickel—a total nucleon number of about 60—is usually an exothermic reaction, exothermic process that releases more energy than is required to bring them together. It is this energy-releasing process that makes nuclear fusion in stars a self-sustaining reaction. For heavier nuclei, the binding energy per nucleon
In physics and chemistry, a nucleon is either a proton or a neutron, considered in its role as a component of an atomic nucleus. The number of nucleons in a nucleus defines the atom's mass number (nucleon number).
Until the 1960s, nucleons w ...
in the nucleus begins to decrease. That means fusion processes producing nuclei that have atomic numbers higher than about 26, and atomic mass
The atomic mass (''m''a or ''m'') is the mass of an atom. Although the SI unit of mass is the kilogram (symbol: kg), atomic mass is often expressed in the non-SI unit dalton (symbol: Da) – equivalently, unified atomic mass unit (u). 1&nbs ...
es higher than about 60, is an endothermic reaction, endothermic process. These more massive nuclei can not undergo an energy-producing fusion reaction that can sustain the hydrostatic equilibrium of a star.
Electron cloud
electromagnetic force
In physics, electromagnetism is an interaction that occurs between particles with electric charge. It is the second-strongest of the four fundamental interactions, after the strong force, and it is the dominant force in the interactions o ...
. This force binds the electrons inside an electrostatic potential well surrounding the smaller nucleus, which means that an external source of energy is needed for the electron to escape. The closer an electron is to the nucleus, the greater the attractive force. Hence electrons bound near the center of the potential well require more energy to escape than those at greater separations.
Electrons, like other particles, have properties of both a wave–particle duality, particle and a wave. The electron cloud is a region inside the potential well where each electron forms a type of three-dimensional standing wave—a wave form that does not move relative to the nucleus. This behavior is defined by an atomic orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in an ...
, a mathematical function that characterises the probability that an electron appears to be at a particular location when its position is measured. Only a discrete (or wikt:quantize, quantized) set of these orbitals exist around the nucleus, as other possible wave patterns rapidly decay into a more stable form. Orbitals can have one or more ring or node structures, and differ from each other in size, shape and orientation.
 Each atomic orbital corresponds to a particular energy level of the electron. The electron can change its state to a higher energy level by absorbing a photon with sufficient energy to boost it into the new quantum state. Likewise, through spontaneous emission, an electron in a higher energy state can drop to a lower energy state while radiating the excess energy as a photon. These characteristic energy values, defined by the differences in the energies of the quantum states, are responsible for atomic spectral lines.
The amount of energy needed to remove or add an electron—the electron binding energy—is far less than the binding energy, binding energy of nucleons. For example, it requires only 13.6 eV to strip a Stationary state, ground-state electron from a hydrogen atom, compared to 2.23 ''million'' eV for splitting a deuterium nucleus. Atoms are electric charge, electrically neutral if they have an equal number of protons and electrons. Atoms that have either a deficit or a surplus of electrons are called
Each atomic orbital corresponds to a particular energy level of the electron. The electron can change its state to a higher energy level by absorbing a photon with sufficient energy to boost it into the new quantum state. Likewise, through spontaneous emission, an electron in a higher energy state can drop to a lower energy state while radiating the excess energy as a photon. These characteristic energy values, defined by the differences in the energies of the quantum states, are responsible for atomic spectral lines.
The amount of energy needed to remove or add an electron—the electron binding energy—is far less than the binding energy, binding energy of nucleons. For example, it requires only 13.6 eV to strip a Stationary state, ground-state electron from a hydrogen atom, compared to 2.23 ''million'' eV for splitting a deuterium nucleus. Atoms are electric charge, electrically neutral if they have an equal number of protons and electrons. Atoms that have either a deficit or a surplus of electrons are called ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
s. Electrons that are farthest from the nucleus may be transferred to other nearby atoms or shared between atoms. By this mechanism, atoms are able to chemical bond, bond into molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bio ...
s and other types of chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one ele ...
s like Ionic crystal, ionic and Covalent bond, covalent network Crystallization, crystals.
Properties
Nuclear properties
By definition, any two atoms with an identical number of ''protons'' in their nuclei belong to the samechemical element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler sub ...
. Atoms with equal numbers of protons but a different number of ''neutrons'' are different isotopes of the same element. For example, all hydrogen atoms admit exactly one proton, but isotopes exist with no neutrons (hydrogen-1, by far the most common form, also called protium), one neutron (deuterium), two neutrons (tritium) and isotopes of hydrogen, more than two neutrons. The known elements form a set of atomic numbers, from the single-proton element hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
up to the 118-proton element oganesson. All known isotopes of elements with atomic numbers greater than 82 are radioactive, although the radioactivity of element 83 (bismuth) is so slight as to be practically negligible.
About 339 nuclides occur naturally on Earth, of which 251 (about 74%) have not been observed to decay, and are referred to as "stable isotope
The term stable isotope has a meaning similar to stable nuclide, but is preferably used when speaking of nuclides of a specific element. Hence, the plural form stable isotopes usually refers to isotopes of the same element. The relative abundanc ...
s". Only 90 nuclides are stable list of nuclides, theoretically, while another 161 (bringing the total to 251) have not been observed to decay, even though in theory it is energetically possible. These are also formally classified as "stable". An additional 35 radioactive nuclides have half-lives longer than 100 million years, and are long-lived enough to have been present since the birth of the Solar System. This collection of 286 nuclides are known as primordial nuclides. Finally, an additional 53 short-lived nuclides are known to occur naturally, as daughter products of primordial nuclide decay (such as radium
Radium is a chemical element with the symbol Ra and atomic number 88. It is the sixth element in group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is silvery-white, but it readily reacts with nitrogen (rathe ...
from uranium), or as products of natural energetic processes on Earth, such as cosmic ray bombardment (for example, carbon-14).For more recent updates see Brookhaven National Laboratory'Interactive Chart of Nuclides
] . For 80 of the chemical elements, at least one
stable isotope
The term stable isotope has a meaning similar to stable nuclide, but is preferably used when speaking of nuclides of a specific element. Hence, the plural form stable isotopes usually refers to isotopes of the same element. The relative abundanc ...
exists. As a rule, there is only a handful of stable isotopes for each of these elements, the average being 3.1 stable isotopes per element. Twenty-six "monoisotopic elements" have only a single stable isotope, while the largest number of stable isotopes observed for any element is ten, for the element tin. Elements technetium, 43, promethium, 61, and all elements numbered bismuth, 83 or higher have no stable isotopes.CRC Handbook (2002).
Stability of isotopes is affected by the ratio of protons to neutrons, and also by the presence of certain "magic numbers" of neutrons or protons that represent closed and filled quantum shells. These quantum shells correspond to a set of energy levels within the Nuclear shell model, shell model of the nucleus; filled shells, such as the filled shell of 50 protons for tin, confers unusual stability on the nuclide. Of the 251 known stable nuclides, only four have both an odd number of protons ''and'' odd number of neutrons: hydrogen-2 (deuterium), lithium-6, boron-10, and nitrogen-14. (Tantalum-180m is odd-odd and observationally stable, but is predicted to decay with a very long half-life.) Also, only four naturally occurring, radioactive odd-odd nuclides have a half-life over a billion years: potassium-40, vanadium-50, lanthanum-138, and lutetium-176. Most odd-odd nuclei are highly unstable with respect to beta decay, because the decay products are even-even, and are therefore more strongly bound, due to Semi-empirical mass formula#Pairing term, nuclear pairing effects.
Mass
The large majority of an atom's mass comes from the protons and neutrons that make it up. The total number of these particles (called "nucleons") in a given atom is called the mass number. It is a positive integer and dimensionless (instead of having dimension of mass), because it expresses a count. An example of use of a mass number is "carbon-12," which has 12 nucleons (six protons and six neutrons). The actual Invariant mass, mass of an atom at rest is often expressed in dalton (unit), daltons (Da), also called the unified atomic mass unit (u). This unit is defined as a twelfth of the mass of a free neutral atom of carbon-12, which is approximately . hydrogen atom, Hydrogen-1 (the lightest isotope of hydrogen which is also the nuclide with the lowest mass) has an atomic weight of 1.007825 Da. The value of this number is called theatomic mass
The atomic mass (''m''a or ''m'') is the mass of an atom. Although the SI unit of mass is the kilogram (symbol: kg), atomic mass is often expressed in the non-SI unit dalton (symbol: Da) – equivalently, unified atomic mass unit (u). 1&nbs ...
. A given atom has an atomic mass approximately equal (within 1%) to its mass number times the atomic mass unit (for example the mass of a nitrogen-14 is roughly 14 Da), but this number will not be exactly an integer except (by definition) in the case of carbon-12. The heaviest stable atom is lead-208, with a mass of .
As even the most massive atoms are far too light to work with directly, chemists instead use the unit of Mole (unit), moles. One mole of atoms of any element always has the same number of atoms (about Avogadro constant, ). This number was chosen so that if an element has an atomic mass of 1 u, a mole of atoms of that element has a mass close to one gram. Because of the definition of the Atomic mass unit, unified atomic mass unit, each carbon-12 atom has an atomic mass of exactly 12 Da, and so a mole of carbon-12 atoms weighs exactly 0.012 kg.
Shape and size
Atoms lack a well-defined outer boundary, so their dimensions are usually described in terms of an atomic radius. This is a measure of the distance out to which the electron cloud extends from the nucleus. This assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. Atomic radii may be derived from the distances between two nuclei when the two atoms are joined in achemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing o ...
. The radius varies with the location of an atom on the atomic chart, the type of chemical bond, the number of neighboring atoms (coordination number) and a quantum mechanics, quantum mechanical property known as Spin (physics), spin. On the periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ...
of the elements, atom size tends to increase when moving down columns, but decrease when moving across rows (left to right). Consequently, the smallest atom is helium with a radius of 32 Picometre, pm, while one of the largest is caesium at 225 pm.
When subjected to external forces, like electrical fields, the shape of an atom may deviate from spherical symmetry. The deformation depends on the field magnitude and the orbital type of outer shell electrons, as shown by group theory, group-theoretical considerations. Aspherical deviations might be elicited for instance in crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macr ...
s, where large crystal-electrical fields may occur at crystal symmetry, low-symmetry lattice sites. Significant ellipsoidal deformations have been shown to occur for sulfur ions and chalcogen ions in pyrite-type compounds.
Atomic dimensions are thousands of times smaller than the wavelengths of light (400–700 nanometre, nm) so they cannot be viewed using an optical microscope, although individual atoms can be observed using a scanning tunneling microscope. To visualize the minuteness of the atom, consider that a typical human hair is about 1 million carbon atoms in width. A single drop of water contains about 2 sextillion () atoms of oxygen, and twice the number of hydrogen atoms. A single Carat (unit), carat diamond with a mass of contains about 10 sextillion (1022) atoms of carbon.A carat is 200 milligrams. Atomic mass unit, By definition, carbon-12 has 0.012 kg per mole. The Avogadro constant defines atoms per mole. If an apple were magnified to the size of the Earth, then the atoms in the apple would be approximately the size of the original apple.
Radioactive decay
atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every ...
.
* Beta decay (and electron capture): these processes are regulated by the weak force, and result from a transformation of a neutron into a proton, or a proton into a neutron. The neutron to proton transition is accompanied by the emission of an electron and an antineutrino, while proton to neutron transition (except in electron capture) causes the emission of a positron and a neutrino
A neutrino ( ; denoted by the Greek letter ) is a fermion (an elementary particle with spin of ) that interacts only via the weak interaction and gravity. The neutrino is so named because it is electrically neutral and because its rest mass ...
. The electron or positron emissions are called beta particles. Beta decay either increases or decreases the atomic number of the nucleus by one. Electron capture is more common than positron emission, because it requires less energy. In this type of decay, an electron is absorbed by the nucleus, rather than a positron emitted from the nucleus. A neutrino is still emitted in this process, and a proton changes to a neutron.
* Gamma decay: this process results from a change in the energy level of the nucleus to a lower state, resulting in the emission of electromagnetic radiation. The excited state of a nucleus which results in gamma emission usually occurs following the emission of an alpha or a beta particle. Thus, gamma decay usually follows alpha or beta decay.
Other more rare types of radioactive decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consid ...
include ejection of neutrons or protons or clusters of nucleon
In physics and chemistry, a nucleon is either a proton or a neutron, considered in its role as a component of an atomic nucleus. The number of nucleons in a nucleus defines the atom's mass number (nucleon number).
Until the 1960s, nucleons w ...
s from a nucleus, or more than one beta particle. An analog of gamma emission which allows excited nuclei to lose energy in a different way, is internal conversion—a process that produces high-speed electrons that are not beta rays, followed by production of high-energy photons that are not gamma rays. A few large nuclei explode into two or more charged fragments of varying masses plus several neutrons, in a decay called spontaneous nuclear fission.
Each radioactive isotope has a characteristic decay time period—the half-life—that is determined by the amount of time needed for half of a sample to decay. This is an exponential decay process that steadily decreases the proportion of the remaining isotope by 50% every half-life. Hence after two half-lives have passed only 25% of the isotope is present, and so forth.
Magnetic moment
Elementary particles possess an intrinsic quantum mechanical property known as Spin (physics), spin. This is analogous to the angular momentum of an object that is spinning around its center of mass, although strictly speaking these particles are believed to be point-like and cannot be said to be rotating. Spin is measured in units of the reduced Planck constant (ħ), with electrons, protons and neutrons all having spin ħ, or "spin-". In an atom, electrons in motion around the Atomic nucleus, nucleus possess orbital angular momentum in addition to their spin, while the nucleus itself possesses angular momentum due to its nuclear spin. The magnetic field produced by an atom—its magnetic moment—is determined by these various forms of angular momentum, just as a rotating charged object classically produces a magnetic field, but the most dominant contribution comes from electron spin. Due to the nature of electrons to obey the Pauli exclusion principle, in which no two electrons may be found in the same quantum state, bound electrons pair up with each other, with one member of each pair in a spin up state and the other in the opposite, spin down state. Thus these spins cancel each other out, reducing the total magnetic dipole moment to zero in some atoms with even number of electrons. In Ferromagnetism, ferromagnetic elements such as iron, cobalt and nickel, an odd number of electrons leads to an unpaired electron and a net overall magnetic moment. The orbitals of neighboring atoms overlap and a lower energy state is achieved when the spins of unpaired electrons are aligned with each other, a spontaneous process known as an exchange interaction. When the magnetic moments of ferromagnetic atoms are lined up, the material can produce a measurable macroscopic field. Paramagnetism, Paramagnetic materials have atoms with magnetic moments that line up in random directions when no magnetic field is present, but the magnetic moments of the individual atoms line up in the presence of a field. The nucleus of an atom will have no spin when it has even numbers of both neutrons and protons, but for other cases of odd numbers, the nucleus may have a spin. Normally nuclei with spin are aligned in random directions because of thermal equilibrium, but for certain elements (such as xenon, xenon-129) it is possible to spin polarization, polarize a significant proportion of the nuclear spin states so that they are aligned in the same direction—a condition called hyperpolarization (physics), hyperpolarization. This has important applications in magnetic resonance imaging.Energy levels
Niels Bohr
Niels Henrik David Bohr (; 7 October 1885 – 18 November 1962) was a Danish physicist who made foundational contributions to understanding atomic structure and quantum theory, for which he received the Nobel Prize in Physics in 1922 ...
model, what can be precisely calculated by the Schrödinger equation
The Schrödinger equation is a linear partial differential equation that governs the wave function of a quantum-mechanical system. It is a key result in quantum mechanics, and its discovery was a significant landmark in the development of th ...
.
Electrons jump between orbitals in a particle-like fashion. For example, if a single photon strikes the electrons, only a single electron changes states in response to the photon; see Atomic orbital, Electron properties.
The energy of an emitted photon is proportional to its frequency, so these specific energy levels appear as distinct bands in the electromagnetic spectrum. Each element has a characteristic spectrum that can depend on the nuclear charge, subshells filled by electrons, the electromagnetic interactions between the electrons and other factors.
Valence and bonding behavior
Valency is the combining power of an element. It is determined by the number of bonds it can form to other atoms or groups. The outermost electron shell of an atom in its uncombined state is known as the valence shell, and the electrons in that shell are called valence electrons. The number of valence electrons determines the chemical bond, bonding behavior with other atoms. Atoms tend to Chemical reaction, chemically react with each other in a manner that fills (or empties) their outer valence shells. For example, a transfer of a single electron between atoms is a useful approximation for bonds that form between atoms with one-electron more than a filled shell, and others that are one-electron short of a full shell, such as occurs in the compound sodium chloride and other chemical ionic salts. Many elements display multiple valences, or tendencies to share differing numbers of electrons in different compounds. Thus,chemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing o ...
ing between these elements takes many forms of electron-sharing that are more than simple electron transfers. Examples include the element carbon and the organic compounds.
The chemical element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler sub ...
s are often displayed in a periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ...
that is laid out to display recurring chemical properties, and elements with the same number of valence electrons form a group that is aligned in the same column of the table. (The horizontal rows correspond to the filling of a quantum shell of electrons.) The elements at the far right of the table have their outer shell completely filled with electrons, which results in chemically inert elements known as the noble gases.
States
 Quantities of atoms are found in different states of matter that depend on the physical conditions, such as temperature and pressure. By varying the conditions, materials can transition between solids, liquids, gases and plasma (physics), plasmas. Within a state, a material can also exist in different allotropes. An example of this is solid carbon, which can exist as graphite or diamond. Gaseous allotropes exist as well, such as dioxygen and ozone.
At temperatures close to absolute zero, atoms can form a Bose–Einstein condensate, at which point quantum mechanical effects, which are normally only observed at the atomic scale, become apparent on a macroscopic scale. This super-cooled collection of atoms
then behaves as a single super atom, which may allow fundamental checks of quantum mechanical behavior.
Quantities of atoms are found in different states of matter that depend on the physical conditions, such as temperature and pressure. By varying the conditions, materials can transition between solids, liquids, gases and plasma (physics), plasmas. Within a state, a material can also exist in different allotropes. An example of this is solid carbon, which can exist as graphite or diamond. Gaseous allotropes exist as well, such as dioxygen and ozone.
At temperatures close to absolute zero, atoms can form a Bose–Einstein condensate, at which point quantum mechanical effects, which are normally only observed at the atomic scale, become apparent on a macroscopic scale. This super-cooled collection of atoms
then behaves as a single super atom, which may allow fundamental checks of quantum mechanical behavior.
Identification
ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
ized by removing one of its electrons, its trajectory when it passes through a magnetic field will bend. The radius by which the trajectory of a moving ion is turned by the magnetic field is determined by the mass of the atom. The Mass spectrometry, mass spectrometer uses this principle to measure the mass-to-charge ratio of ions. If a sample contains multiple isotopes, the mass spectrometer can determine the proportion of each isotope in the sample by measuring the intensity of the different beams of ions. Techniques to vaporize atoms include inductively coupled plasma atomic emission spectroscopy and inductively coupled plasma mass spectrometry, both of which use a plasma to vaporize samples for analysis.
The atom probe, atom-probe tomograph has sub-nanometer resolution in 3-D and can chemically identify individual atoms using time-of-flight mass spectrometry.
Electron emission techniques such as X-ray photoelectron spectroscopy (XPS) and Auger electron spectroscopy (AES), which measure the binding energies of the core electrons, are used to identify the atomic species present in a sample in a non-destructive way. With proper focusing both can be made area-specific. Another such method is electron energy loss spectroscopy (EELS), which measures the energy loss of an electron beam within a transmission electron microscope when it interacts with a portion of a sample.
Spectra of excited states can be used to analyze the atomic composition of distant stars. Specific light wavelengths contained in the observed light from stars can be separated out and related to the quantized transitions in free gas atoms. These colors can be replicated using a gas-discharge lamp containing the same element. Helium was discovered in this way in the spectrum of the Sun 23 years before it was found on Earth.
Origin and current state
Baryonic matter forms about 4% of the total energy density of the observable universe, with an average density of about 0.25 particles/m3 (mostly protons and electrons). Within a galaxy such as the Milky Way, particles have a much higher concentration, with the density of matter in the interstellar medium (ISM) ranging from 105 to 109 atoms/m3. The Sun is believed to be inside the Local Bubble, so the density in the solar neighborhood is only about 103 atoms/m3. Stars form from dense clouds in the ISM, and the evolutionary processes of stars result in the steady enrichment of the ISM with elements more massive than hydrogen and helium. Up to 95% of the Milky Way's baryonic matter are concentrated inside stars, where conditions are unfavorable for atomic matter. The total baryonic mass is about 10% of the mass of the galaxy; the remainder of the mass is an unknown dark matter. High temperature inside stars makes most "atoms" fully ionized, that is, separates ''all'' electrons from the nuclei. In stellar remnants—with exception of their surface layers—an immense pressure make electron shells impossible.Formation
Earth
Most of the atoms that make up the Earth and its inhabitants were present in their current form in the nebula that collapsed out of a molecular cloud to form the Solar System. The rest are the result of radioactive decay, and their relative proportion can be used to determine the age of the Earth through radiometric dating.#refManuel2001, Manuel (2001). ''Origin of Elements in the Solar System'', pp. 407-430, 511-519 Most of the helium in the crust of the Earth (about 99% of the helium from gas wells, as shown by its lower abundance of helium-3) is a product of alpha decay. There are a few trace atoms on Earth that were not present at the beginning (i.e., not "primordial"), nor are results of radioactive decay. Carbon-14 is continuously generated by cosmic rays in the atmosphere. Some atoms on Earth have been artificially generated either deliberately or as by-products of nuclear reactors or explosions. Of the Transuranium element, transuranic elements—those with atomic numbers greater than 92—only plutonium and neptunium occur naturally on Earth. Transuranic elements have radioactive lifetimes shorter than the current age of the Earth and thus identifiable quantities of these elements have long since decayed, with the exception of traces of plutonium-244 possibly deposited by cosmic dust. Natural deposits of plutonium and neptunium are produced by neutron capture in uranium ore. The Earth contains approximately atoms. Although small numbers of independent atoms of noble gases exist, such as argon, neon, and helium, 99% of Earth's atmosphere, the atmosphere is bound in the form of molecules, including carbon dioxide and Diatomic molecule, diatomic oxygen and nitrogen. At the surface of the Earth, an overwhelming majority of atoms combine to form various compounds, including water, salt, silicates and oxides. Atoms can also combine to create materials that do not consist of discrete molecules, includingcrystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macr ...
s and liquid or solid metals. This atomic matter forms networked arrangements that lack the particular type of small-scale interrupted order associated with molecular matter.
Rare and theoretical forms
Superheavy elements
All nuclides with atomic numbers higher than 82 (lead) are known to be radioactive. No nuclide with an atomic number exceeding 92 (uranium) exists on Earth as a primordial nuclide, and heavier elements generally have shorter half-lives. Nevertheless, an "island of stability" encompassing relatively long-lived isotopes of superheavy elements with atomic numbers darmstadtium, 110 to flerovium, 114 might exist. Predictions for the half-life of the most stable nuclide on the island range from a few minutes to millions of years. In any case, superheavy elements (with ''Z'' > 104) would not exist due to increasing Coulomb repulsion (which results in spontaneous fission with increasingly short half-lives) in the absence of any stabilizing effects.Exotic matter
Each particle of matter has a corresponding antimatter particle with the opposite electrical charge. Thus, the positron is a positively charged antielectron and the antiproton is a negatively charged equivalent of a proton. When a matter and corresponding antimatter particle meet, they annihilate each other. Because of this, along with an imbalance between the number of matter and antimatter particles, the latter are rare in the universe. The first causes of this imbalance are not yet fully understood, although theories of baryogenesis may offer an explanation. As a result, no antimatter atoms have been discovered in nature. In 1996, the antimatter counterpart of the hydrogen atom (antihydrogen) was synthesized at the CERN laboratory in Geneva. Other exotic atoms have been created by replacing one of the protons, neutrons or electrons with other particles that have the same charge. For example, an electron can be replaced by a more massive muon, forming a muonic atom. These types of atoms can be used to test fundamental predictions of physics.See also
Notes
References
Bibliography
* * * * * * * * *Further reading
* * * * * * *External links
* * {{Authority control Atoms, Chemistry Articles containing video clips