Atactic on:
[Wikipedia]
[Google]
[Amazon]
Tacticity (from , "relating to arrangement or order") is the relative


 :
: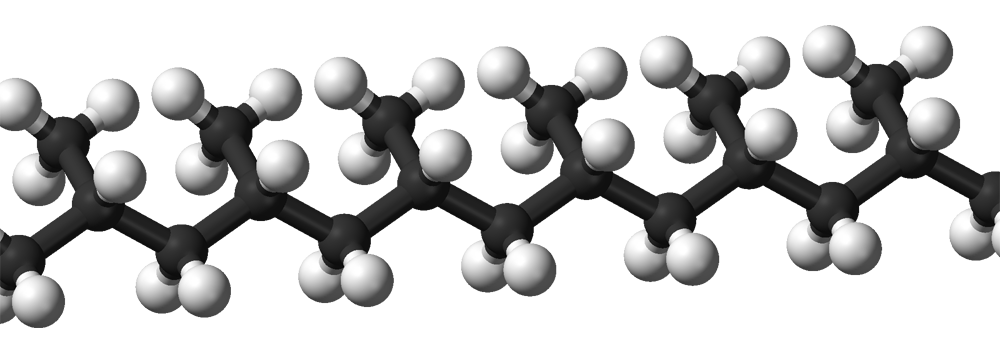
 :
:
 Polymers that are formed by free-radical mechanisms, such as
Polymers that are formed by free-radical mechanisms, such as
Application of spectroscopy in polymer charactisation
@
stereochemistry
Stereochemistry, a subdiscipline of chemistry, studies the spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereoisomers, which are defined ...
of adjacent chiral
Chirality () is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek language, Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is dist ...
centers within a macromolecule
A macromolecule is a "molecule of high relative molecular mass, the structure of which essentially comprises the multiple repetition of units derived, actually or conceptually, from molecules of low relative molecular mass." Polymers are physi ...
. The practical significance of tacticity rests on the effects on the physical properties of the polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
. The regularity of the macromolecular structure influences the degree to which it has rigid, crystalline
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macrosc ...
long range order or flexible, amorphous
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid) is a solid that lacks the long-range order that is a characteristic of a crystal. The terms "glass" and "glassy solid" are sometimes used synonymousl ...
long range disorder. Precise knowledge of tacticity of a polymer also helps understanding at what temperature a polymer melts, how soluble
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubi ...
it is in a solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
, as well as its mechanical properties.
A tactic macromolecule in the IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
definition is a macromolecule in which essentially all the configurational (repeating) units are identical. In a hydrocarbon macromolecule with all carbon atoms making up the backbone in a tetrahedral molecular geometry
In a tetrahedral molecular geometry, a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are arccos(−) = 109.4712206...° ≈ 109.5° when all four substituents are ...
, the zigzag backbone is in the paper plane with the substituents either sticking out of the paper or retreating into the paper;, this projection is called the Natta projection after Giulio Natta. Tacticity is particularly significant in vinyl polymers of the type -, where each repeating unit contains a substituent
In organic chemistry, a substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule.
The suffix ''-yl'' is used when naming organic compounds that contain a single bond r ...
R attached to one side of the polymer backbone. The arrangement of these substituents can follow a regular pattern- appearing on the same side as the previous one, on the opposite side, or in a random configuration relative to the preceding unit. Monotactic macromolecules have one stereoisomeric atom per repeat unit, ditactic to n-tactic macromolecules have more than one stereoisomeric atom per unit.
Definition

Diads
Two adjacent structural units in a polymer molecule constitute a diad. Diads overlap: each structural unit is considered part of two diads, one diad with each neighbor. If a diad consists of two identically oriented units, the diad is called an (formerly ''meso diad'', as in ameso compound
A meso compound or meso isomer is an optically inactive isomer in a set of stereoisomers, at least two of which are optically active. This means that despite containing two or more stereocenters, the molecule is not chiral. A meso compound is su ...
, now proscribed). If a diad consists of units oriented in opposition, the diad is called an (formerly ''racemo diad'', as in a racemic compound, now proscribed). In the case of vinyl polymer molecules, an is one in which the substituents are oriented on the same side of the polymer backbone; in the Natta projection, they both point into the plane or both point out of the plane.
Triads
The stereochemistry of macromolecules can be defined even more precisely with the introduction of triads. An isotactic triad (''mm'') is made up of two adjacent m diads, a syndiotactic triad (also spelled syndyotactic) (''rr'') consists of two adjacent , and a heterotactic triad (''rm'') is composed of an adjacent to an . The mass fraction of isotactic (''mm'') triads is a common quantitative measure of tacticity. When the stereochemistry of a macromolecule is considered to be aBernoulli process
In probability and statistics, a Bernoulli process (named after Jacob Bernoulli) is a finite or infinite sequence of binary random variables, so it is a discrete-time stochastic process that takes only two values, canonically 0 and 1. The ...
, the triad composition can be calculated from the probability ''P''m of a diad being . For example, when this probability is 0.25 then the probability of finding:
*an isotactic triad is ''P''m2, or 0.0625
*an heterotactic triad is 2''P''m(1–''P''m), or 0.375
*a syndiotactic triad is (1–''P''m)2, or 0.5625
with a total probability of 1. Similar relationships with diads exist for tetrads.
Tetrads, pentads, etc.
The definition of tetrads and pentads introduce further sophistication and precision to defining tacticity, especially when information on long-range ordering is desirable. Tacticity measurements obtained bycarbon-13 NMR
Carbon-13 (C13) nuclear magnetic resonance (most commonly known as carbon-13 NMR spectroscopy or 13C NMR spectroscopy or sometimes simply referred to as carbon NMR) is the application of nuclear magnetic resonance (NMR) spectroscopy to carbon. It ...
are typically expressed in terms of the relative abundance of various pentads within the polymer molecule, e.g. ''mmmm'', ''mrrm''.
Other conventions for quantifying tacticity
The primary convention for expressing tacticity is in terms of the relative weight fraction of triad or higher-order components, as described above. An alternative expression for tacticity is the average length of ''m'' and ''r'' sequences within the polymer molecule. The average m-sequence length may be approximated from the relative abundance of pentads as follows:Polymers

Isotactic polymers
Isotactic polymers are composed of isotactic macromolecules (IUPAC definition). In isotactic macromolecules, all the substituents are located on the same side of the macromolecular backbone. An isotactic macromolecule consists of 100% , though IUPAC also allows the term for macromolecules with at least 95% if that looser usage is explained.Polypropylene
Polypropylene (PP), also known as polypropene, is a thermoplastic polymer used in a wide variety of applications. It is produced via chain-growth polymerization from the monomer Propene, propylene.
Polypropylene belongs to the group of polyolefin ...
formed by Ziegler–Natta catalysis is an example of an isotactic polymer. Isotactic polymers are usually semicrystalline
Crystallinity refers to the degree of structural order in a solid. In a crystal, the atoms or molecules are arranged in a regular, periodic manner. The degree of crystallinity has a large influence on hardness, density, Transparency and transluc ...
and often form a helix configuration.
: :
:
Syndiotactic polymers
In syndiotactic or syntactic macromolecules the substituents have alternate positions along the chain. The macromolecule comprises 100% , though IUPAC also allows the term for macromolecules with at least 95% if that looser usage is explained. Syndiotacticpolystyrene
Polystyrene (PS) is a synthetic polymer made from monomers of the aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It i ...
, made by metallocene catalysis polymerization, is crystalline with aa melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state of matter, state from solid to liquid. At the melting point the solid and liquid phase (matter), phase exist in Thermodynamic equilib ...
of 161 °C. Gutta percha is also an example syndiotactic polymer.
: :
:
Atactic polymers
In atactic macromolecules the substituents are placed randomly along the chain. The percentage of is understood to be between 45 and 55% unless otherwise specified, but it could be any value other than 0 or 100% if that usage is clarified. With the aid of spectroscopic techniques such asNMR
Nuclear magnetic resonance (NMR) is a physical phenomenon in which atomic nucleus, nuclei in a strong constant magnetic field are disturbed by a weak oscillating magnetic field (in the near and far field, near field) and respond by producing ...
, it is possible to pinpoint the composition of a polymer in terms of the percentages for each triad.
: Polymers that are formed by free-radical mechanisms, such as
Polymers that are formed by free-radical mechanisms, such as polyvinyl chloride
Polyvinyl chloride (alternatively: poly(vinyl chloride), colloquial: vinyl or polyvinyl; abbreviated: PVC) is the world's third-most widely produced synthetic polymer of plastic (after polyethylene and polypropylene). About 40 million tons of ...
are usually atactic. Due to their random nature atactic polymers are usually amorphous
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid) is a solid that lacks the long-range order that is a characteristic of a crystal. The terms "glass" and "glassy solid" are sometimes used synonymousl ...
. In ''hemi-isotactic macromolecules'' every other repeat unit has a random substituent.
Atactic polymers such as polystyrene
Polystyrene (PS) is a synthetic polymer made from monomers of the aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It i ...
( PS) are technologically very important. It is possible to obtain syndiotactic polystyrene using a Kaminsky catalyst
A Kaminsky catalyst is a catalytic system for alkene polymerization. Kaminsky catalysts are based on metallocenes of group 4 transition metals (Ti, Zr, Hf) activated with methylaluminoxane (MAO). These and other innovations have inspired develop ...
, but most industrial polystyrene produced is atactic. The two materials have very different properties because the irregular structure of the atactic version makes it impossible for the polymer chains to stack in a regular fashion: whereas syndiotactic PS is a semicrystalline material, the more common atactic version cannot crystallize and forms a ''glass'' instead. This example is quite general in that many polymers of economic importance are atactic glass formers.
Eutactic polymers
In eutactic macromolecules, substituents may occupy any specific (but potentially complex) sequence of positions along the chain. Isotactic and syndiotactic polymers are instances of the more general class of eutactic polymers, which also includes heterogeneous macromolecules in which the sequence consists of substituents of different kinds (for example, the side-chains in proteins and the bases in nucleic acids).Head/tail configuration
In vinyl polymers, the complete configuration can be further described by defining polymer head/tail configuration. In a regular macromolecule, monomer units are normally linked in a head to tail configuration such that β-substituents are located on alternating carbon atoms. However, it is possible for defects to form where substituents are placed on adjacent carbon atoms, producing a head/head tail/tail configuration, such as by recombination of two growing radical chains, or by direct head-head addition if steric effects are weak enough, such as inpolyvinylidene fluoride
Polyvinylidene fluoride or polyvinylidene difluoride (PVDF) is a highly non-reactive thermoplastic fluoropolymer produced by the polymerization of vinylidene difluoride. Its chemical formula is (C2H2F2)''n''.
PVDF is a specialty plastic use ...
.
Techniques for measuring tacticity
Tacticity may be measured directly usingproton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
or carbon-13
Carbon-13 (13C) is a natural, stable isotope of carbon with a nucleus containing six protons and seven neutrons. As one of the environmental isotopes, it makes up about 1.1% of all natural carbon on Earth.
Detection by mass spectrometry
A m ...
NMR
Nuclear magnetic resonance (NMR) is a physical phenomenon in which atomic nucleus, nuclei in a strong constant magnetic field are disturbed by a weak oscillating magnetic field (in the near and far field, near field) and respond by producing ...
. This technique enables quantification of the tacticity distribution by comparison of peak areas or integral ranges corresponding to known diads (r, m), triads (mm, rm+mr, rr) and/or higher order ''n''-ads, depending on spectral resolution. In cases of limited resolution, stochastic methods such as Bernoullian or Markovian analysis may also be used to fit the distribution and predict higher ''n''-ads and calculate the isotacticity of the polymer to the desired level.
Other techniques sensitive to tacticity include x-ray powder diffraction, secondary ion mass spectrometry (SIMS), vibrational spectroscopy (FTIR) and especially two-dimensional techniques. Tacticity may also be inferred by measuring another physical property, such as melting temperature, when the relationship between tacticity and that property is well-established.
References
Further reading
* {{cite web , author = Wandrey, Christine rof., date = 2004-04-19 , title = Molecular Basis of the Structure and Behavior of Polymers, Part II: Chemistry and Structure of Macromolecules—Design of Polymer Chains , type = polymer chemistry course materils , work = EPFL.ch , location = Lausanne, Switzerland , publisher = Laboratory of Polymers and Biomaterials, Dept. of Chemistry, Ecole Polytechnique Federale de Lausanne (EPFL) , url = http://scgc.epfl.ch:80/load/cours_chim/cwandrey_part-2-1.pdf , url-status = dead , archive-url = https://web.archive.org/web/20040419014355/http://scgc.epfl.ch:80/load/cours_chim/cwandrey_part-2-1.pdf , archive-date = 2004-04-19External links
Application of spectroscopy in polymer charactisation
@
University of California, Los Angeles
The University of California, Los Angeles (UCLA) is a public university, public Land-grant university, land-grant research university in Los Angeles, California, United States. Its academic roots were established in 1881 as a normal school the ...
Polymer chemistry
Stereochemistry