Antimony Massive on:
[Wikipedia]
[Google]
[Amazon]
Antimony is a

 Antimony is a member of group 15 of the
Antimony is a member of group 15 of the
 The abundance of antimony in the Earth's crust is estimated at 0.2
The abundance of antimony in the Earth's crust is estimated at 0.2
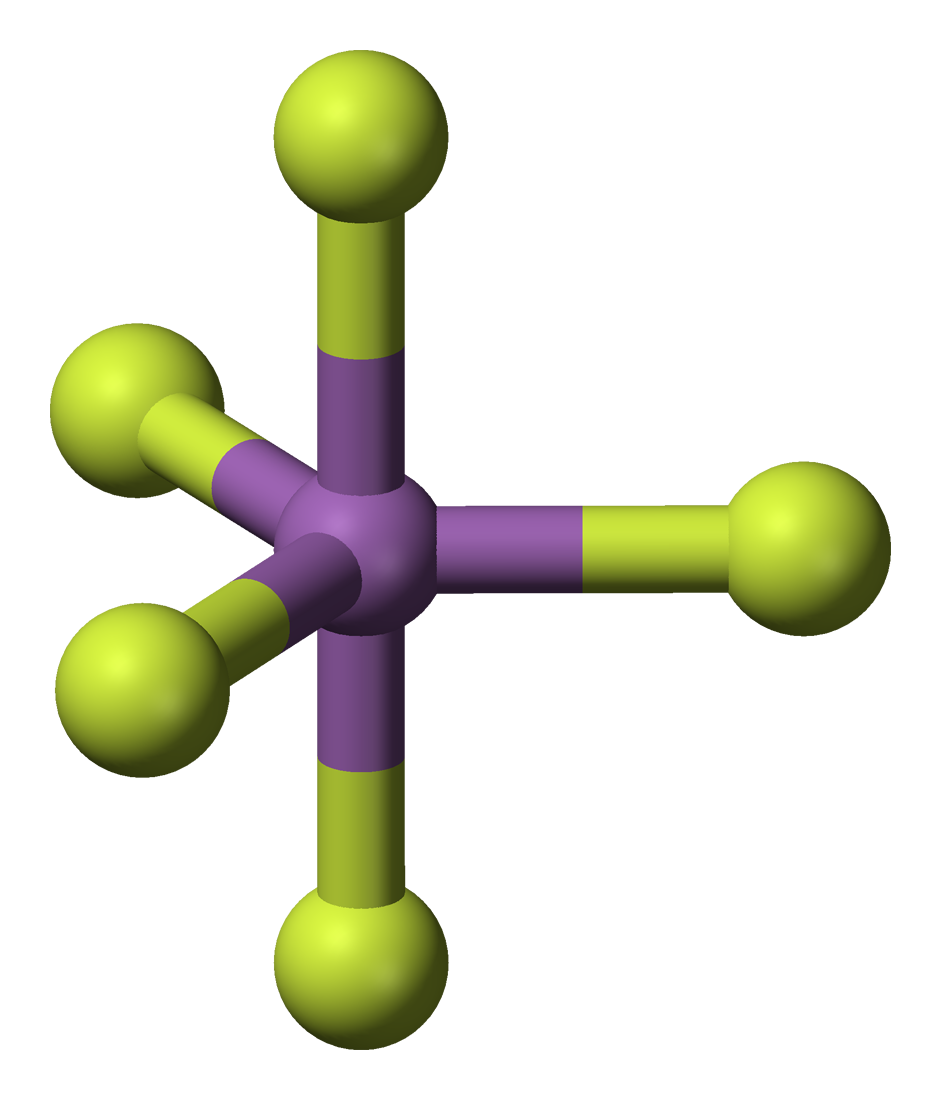 The pentahalides and have trigonal bipyramidal molecular geometry in the gas phase, but in the liquid phase, is
The pentahalides and have trigonal bipyramidal molecular geometry in the gas phase, but in the liquid phase, is


 Three other applications consume nearly all the rest of the world's supply. One application is as a stabilizer and catalyst for the production of polyethylene terephthalate. Another is as a fining agent to remove microscopic bubbles in glass, mostly for TV screens antimony ions interact with oxygen, suppressing the tendency of the latter to form bubbles. The third application is pigments.
In the 1990s antimony was increasingly being used in semiconductors as a
Three other applications consume nearly all the rest of the world's supply. One application is as a stabilizer and catalyst for the production of polyethylene terephthalate. Another is as a fining agent to remove microscopic bubbles in glass, mostly for TV screens antimony ions interact with oxygen, suppressing the tendency of the latter to form bubbles. The third application is pigments.
In the 1990s antimony was increasingly being used in semiconductors as a
Health Canada. July 2020. * EU and German Federal Ministry of Environment: 5 μg/L The tolerable daily intake (TDI) proposed by WHO is 6 μg antimony per kilogram of body weight. The immediately dangerous to life or health (IDLH) value for antimony is 50 mg/m3.
Public Health Statement for Antimony
International Antimony Association vzw (i2a
Chemistry in its element podcast
(MP3) from the Royal Society of Chemistry's Chemistry World
Antimony
at ''The Periodic Table of Videos'' (University of Nottingham)
CDC – NIOSH Pocket Guide to Chemical Hazards – Antimony
Antimony Mineral data and specimen images
{{Authority control Antimony, Chemical elements Metalloids Native element minerals Nuclear materials Pnictogens Trigonal minerals Minerals in space group 166 Chemical elements with rhombohedral structure
chemical element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its ...
; it has symbol
A symbol is a mark, Sign (semiotics), sign, or word that indicates, signifies, or is understood as representing an idea, physical object, object, or wikt:relationship, relationship. Symbols allow people to go beyond what is known or seen by cr ...
Sb () and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
51. A lustrous grey metal or metalloid
A metalloid is a chemical element which has a preponderance of material property, properties in between, or that are a mixture of, those of metals and Nonmetal (chemistry), nonmetals. The word metalloid comes from the Latin language, Latin ''meta ...
, it is found in nature mainly as the sulfide mineral
The sulfide minerals are a class of minerals containing sulfide (S2−) or disulfide () as the major anion. Some sulfide minerals are economically important as metal ores. The sulfide class also includes the selenide mineral, selenides, the tell ...
stibnite (). Antimony compounds have been known since ancient times and were powdered for use as medicine and cosmetics, often known by the Arabic name kohl. The earliest known description of this metalloid in the West was written in 1540 by Vannoccio Biringuccio.
China is the largest producer of antimony and its compounds, with most production coming from the Xikuangshan Mine in Hunan. The industrial methods for refining antimony from stibnite are roasting
Roasting is a cooking method that uses dry heat where hot air covers the food, cooking it evenly on all sides with temperatures of at least from an open flame, oven, or other heat source. Roasting can enhance the flavor through caramelizat ...
followed by reduction with carbon, or direct reduction of stibnite with iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
.
The most common applications for metallic antimony are in alloy
An alloy is a mixture of chemical elements of which in most cases at least one is a metal, metallic element, although it is also sometimes used for mixtures of elements; herein only metallic alloys are described. Metallic alloys often have prop ...
s with lead and tin, which have improved properties for solder
Solder (; North American English, NA: ) is a fusible alloy, fusible metal alloy used to create a permanent bond between metal workpieces. Solder is melted in order to wet the parts of the joint, where it adheres to and connects the pieces aft ...
s, bullets
A bullet is a Kinetic energy weapon, kinetic projectile, a component of firearm ammunition that is Shooting, shot from a gun barrel. They are made of a variety of materials, such as copper, lead, steel, polymer, rubber and even wax; and are made ...
, and plain bearing
file:NYC 100-driving-axle-friction-bearing.jpg, Plain bearing on a 1906 S-Motor locomotive showing the axle, bearing, oil supply and oiling pad
file:Linear-table with detail numbered.png, A sliding table with four cylindrical bearings
file:GWR Spo ...
s. It improves the rigidity of lead-alloy plates in lead–acid batteries. Antimony trioxide is a prominent additive for halogen
The halogens () are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would ...
-containing flame retardant
Flame retardants are a diverse group of chemicals that are added to manufactured materials, such as plastics and textiles, and surface finishes and coatings. Flame retardants are activated by the presence of an combustion, ignition source and pr ...
s. Antimony is used as a dopant
A dopant (also called a doping agent) is a small amount of a substance added to a material to alter its physical properties, such as electrical or optics, optical properties. The amount of dopant is typically very low compared to the material b ...
in semiconductor device
A semiconductor device is an electronic component that relies on the electronic properties of a semiconductor material (primarily silicon, germanium, and gallium arsenide, as well as organic semiconductors) for its function. Its conductivit ...
s.
Characteristics
Properties
 Antimony is a member of group 15 of the
Antimony is a member of group 15 of the periodic table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other s ...
, one of the elements called pnictogen
, -
! colspan=2 style="text-align:left;" , ↓ Period
, -
! 2
,
, -
! 3
,
, -
! 4
,
, -
! 5
,
, -
! 6
,
, -
! 7
,
, -
, colspan="2",
----
''Legend''
A pnictogen ( or ; from "to choke" and -gen, "generator") is any ...
s, and has an electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
of 2.05. In accordance with periodic trends, it is more electronegative than tin or bismuth
Bismuth is a chemical element; it has symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs nat ...
, and less electronegative than tellurium
Tellurium is a chemical element; it has symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionally fou ...
or arsenic
Arsenic is a chemical element; it has Symbol (chemistry), symbol As and atomic number 33. It is a metalloid and one of the pnictogens, and therefore shares many properties with its group 15 neighbors phosphorus and antimony. Arsenic is not ...
. Antimony is stable in air at room temperature but, if heated, it reacts with oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
to produce antimony trioxide,. Wiberg and Holleman, p. 758
Antimony is a silvery, lustrous gray metalloid with a Mohs scale
The Mohs scale ( ) of mineral hardness is a qualitative ordinal scale, from 1 to 10, characterizing scratch resistance of minerals through the ability of harder material to scratch softer material.
The scale was introduced in 1812 by the Ger ...
hardness of 3, which is too soft to mark hard objects. Coins of antimony were issued in China's Guizhou
)
, image_skyline =
, image_caption =
, image_map = Guizhou in China (+all claims hatched).svg
, mapsize = 275px
, map_alt = Map showing the location of Guizhou Province
, map_caption = Map s ...
in 1931; durability was poor, and minting was soon discontinued because of its softness and toxicity. Antimony is resistant to attack by acids.
The only stable allotrope of antimony under standard conditions is metallic, brittle
A material is brittle if, when subjected to stress, it fractures with little elastic deformation and without significant plastic deformation. Brittle materials absorb relatively little energy prior to fracture, even those of high strength. ...
, silver-white, and shiny. It crystallises in a trigonal
In crystallography, the hexagonal crystal family is one of the six crystal family, crystal families, which includes two crystal systems (hexagonal and trigonal) and two lattice systems (hexagonal and rhombohedral). While commonly confused, the tr ...
cell, isomorphic
In mathematics, an isomorphism is a structure-preserving mapping or morphism between two structures of the same type that can be reversed by an inverse mapping. Two mathematical structures are isomorphic if an isomorphism exists between the ...
with bismuth
Bismuth is a chemical element; it has symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs nat ...
and the gray allotrope of arsenic
Arsenic is a chemical element; it has Symbol (chemistry), symbol As and atomic number 33. It is a metalloid and one of the pnictogens, and therefore shares many properties with its group 15 neighbors phosphorus and antimony. Arsenic is not ...
, and is formed when molten antimony is cooled slowly. Amorphous black antimony is formed upon rapid cooling of antimony vapor, and is only stable as a thin film (thickness in nanometres); thicker samples spontaneously transform into the metallic form. It oxidizes in air and may ignite spontaneously. At 100 °C, it gradually transforms into the stable form. The supposed yellow allotrope of antimony, generated only by oxidation of stibine () at −90 °C, is also impure and not a true allotrope; above this temperature and in ambient light, it transforms into the more stable black allotrope. A rare explosive form of antimony can be formed from the electrolysis of antimony trichloride, but it always contains appreciable chlorine and is not really an antimony allotrope. When scratched with a sharp implement, an exothermic reaction occurs and white fumes are given off as metallic antimony forms; when rubbed with a pestle in a mortar, a strong detonation occurs.
Elemental antimony adopts a layered structure (space group
In mathematics, physics and chemistry, a space group is the symmetry group of a repeating pattern in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of the pattern that ...
Rm No. 166) whose layers consist of fused, ruffled, six-membered rings. The nearest and next-nearest neighbors form an irregular octahedral complex, with the three atoms in each double layer slightly closer than the three atoms in the next. This relatively close packing leads to a high density of 6.697 g/cm3, but the weak bonding between the layers leads to the low hardness and brittleness of antimony.
Isotopes
Antimony has two stableisotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
s: with a natural abundance of 57.36% and with a natural abundance of 42.64%. It also has 35 radioisotopes, of which the longest-lived is with a half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
of 2.75 years. In addition, 29 metastable
In chemistry and physics, metastability is an intermediate energetic state within a dynamical system other than the system's state of least energy.
A ball resting in a hollow on a slope is a simple example of metastability. If the ball is onl ...
states have been characterized. The most stable of these is with a half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
of 5.76 days. Isotopes that are lighter than the stable tend to decay by β+ decay, and those that are heavier tend to decay by β− decay, with some exceptions. Antimony is the lightest element to have an isotope with an alpha decay branch, excluding and other light nuclides with beta-delayed alpha emission.
Occurrence
 The abundance of antimony in the Earth's crust is estimated at 0.2
The abundance of antimony in the Earth's crust is estimated at 0.2 parts per million
In science and engineering, the parts-per notation is a set of pseudo-units to describe the small values of miscellaneous dimensionless quantity, dimensionless quantities, e.g. mole fraction or mass fraction (chemistry), mass fraction.
Since t ...
, Greenwood and Earnshaw, p. 548 comparable to thallium
Thallium is a chemical element; it has Symbol (chemistry), symbol Tl and atomic number 81. It is a silvery-white post-transition metal that is not found free in nature. When isolated, thallium resembles tin, but discolors when exposed to air. Che ...
at 0.5 ppm and silver at 0.07 ppm. It is the 63rd most abundant element in the crust. Even though this element is not abundant, it is found in more than 100 mineral species. Antimony is sometimes found natively (e.g. on Antimony Peak), but more frequently it is found in the sulfide stibnite () which is the predominant ore mineral.
Compounds
Antimony compounds are often classified according to their oxidation state: Sb(III) and Sb(V). The +5oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
is more common.
Oxides and hydroxides
Antimony trioxide is formed when antimony is burnt in air. In the gas phase, the molecule of the compound is , but it polymerizes upon condensing. Antimony pentoxide () can be formed only by oxidation with concentratednitric acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most com ...
. Antimony also forms a mixed-valence oxide, antimony tetroxide (), which features both Sb(III) and Sb(V). Unlike oxides of phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ...
and arsenic
Arsenic is a chemical element; it has Symbol (chemistry), symbol As and atomic number 33. It is a metalloid and one of the pnictogens, and therefore shares many properties with its group 15 neighbors phosphorus and antimony. Arsenic is not ...
, these oxides are amphoteric, do not form well-defined oxoacids, and react with acids to form antimony salts.
Antimonous acid is unknown, but the conjugate base sodium antimonite () forms upon fusing sodium oxide and . Transition metal antimonites are also known. Antimonic acid exists only as the hydrate , forming salts as the antimonate anion . When a solution containing this anion is dehydrated, the precipitate contains mixed oxides.
The most important antimony ore is stibnite (). Other sulfide minerals include pyrargyrite (), zinkenite, jamesonite, and boulangerite. Antimony pentasulfide is non-stoichiometric
Non-stoichiometric compounds are chemical compounds, almost always solid inorganic compounds, having chemical element, elemental composition whose proportions cannot be represented by a ratio of small natural numbers (i.e. an empirical formula); ...
, which features antimony in the +3 oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
and S–S bonds. Several thioantimonides are known, such as and .
Halides
Antimony forms two series of halides: and . The trihalides , , , and are all molecular compounds having trigonal pyramidal molecular geometry. The trifluoride is prepared by the reaction of antimony trioxide withhydrofluoric acid
Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colorless, acidic and highly corrosive. A common concentration is 49% (48–52%) but there are also stronger solutions (e.g. 70%) and pure HF has a boiling p ...
:
:
It is Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any ...
ic and readily accepts fluoride ions to form the complex anions and . Molten antimony trifluoride is a weak electrical conductor
In physics and electrical engineering, a conductor is an object or type of material that allows the flow of charge (electric current) in one or more directions. Materials made of metal are common electrical conductors. The flow of negatively c ...
. The trichloride is prepared by dissolving stibnite in hydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
:
:
Arsenic sulfides are not readily attacked by the hydrochloric acid, so this method offers a route to As-free Sb.
 The pentahalides and have trigonal bipyramidal molecular geometry in the gas phase, but in the liquid phase, is
The pentahalides and have trigonal bipyramidal molecular geometry in the gas phase, but in the liquid phase, is polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
ic, whereas is monomeric. Antimony pentafluoride is a powerful Lewis acid used to make the superacid
In chemistry, a superacid (according to the original definition) is an acid with an acidity greater than that of 100% pure sulfuric acid (), which has a Hammett acidity function (''H''0) of −12. According to the modern definition, a superacid i ...
fluoroantimonic acid ().
Oxyhalides are more common for antimony than for arsenic and phosphorus. Antimony trioxide dissolves in concentrated acid to form oxoantimonyl compounds such as SbOCl and .
Antimonides, hydrides, and organoantimony compounds
Compounds in this class generally are described as derivatives of . Antimony forms antimonides with metals, such as indium antimonide (InSb) and silver antimonide (). The alkali metal and zinc antimonides, such as and , are more reactive. Treating these antimonides with acid produces the highly unstable gas stibine, : : Stibine can also be produced by treating salts with hydride reagents such assodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate and sodium tetrahydroborate, is an inorganic compound with the formula (sometimes written as ). It is a white crystalline solid, usually encountered as an aqueous basic solution. Sodi ...
. Stibine decomposes spontaneously at room temperature. Because stibine has a positive heat of formation, it is thermodynamically unstable and thus antimony does not react with hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
directly.
Organoantimony compounds are typically prepared by alkylation of antimony halides with Grignard reagent
Grignard reagents or Grignard compounds are chemical compounds with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromi ...
s. A large variety of compounds are known with both Sb(III) and Sb(V) centers, including mixed chloro-organic derivatives, anions, and cations. Examples include triphenylstibine () and pentaphenylantimony ().
History
Antimony(III) sulfide, , was recognized in predynastic Egypt as an eye cosmetic ( kohl) as early as about 3100 BC, when the cosmetic palette was invented. An artifact, said to be part of a vase, made of antimony dating to about 3000 BC was found at Telloh,Chaldea
Chaldea () refers to a region probably located in the marshy land of southern Mesopotamia. It is mentioned, with varying meaning, in Neo-Assyrian cuneiform, the Hebrew Bible, and in classical Greek texts. The Hebrew Bible uses the term (''Ka� ...
(part of present-day Iraq
Iraq, officially the Republic of Iraq, is a country in West Asia. It is bordered by Saudi Arabia to Iraq–Saudi Arabia border, the south, Turkey to Iraq–Turkey border, the north, Iran to Iran–Iraq border, the east, the Persian Gulf and ...
), and a copper object plated with antimony dating between 2500 BC and 2200 BC has been found in Egypt
Egypt ( , ), officially the Arab Republic of Egypt, is a country spanning the Northeast Africa, northeast corner of Africa and Western Asia, southwest corner of Asia via the Sinai Peninsula. It is bordered by the Mediterranean Sea to northe ...
. Austen, at a lecture by Herbert Gladstone in 1892, commented that "we only know of antimony at the present day as a highly brittle and crystalline metal, which could hardly be fashioned into a useful vase, and therefore this remarkable 'find' (artifact mentioned above) must represent the lost art of rendering antimony malleable."
The British archaeologist Roger Moorey was unconvinced the artifact was indeed a vase, mentioning that Selimkhanov, after his analysis of the Tello object (published in 1975), "attempted to relate the metal to Transcaucasian natural antimony" (i.e. native metal) and that "the antimony objects from Transcaucasia are all small personal ornaments." This weakens the evidence for a lost art "of rendering antimony malleable".
The Roman scholar Pliny the Elder
Gaius Plinius Secundus (AD 23/24 79), known in English as Pliny the Elder ( ), was a Roman Empire, Roman author, Natural history, naturalist, and naval and army commander of the early Roman Empire, and a friend of the Roman emperor, emperor Vesp ...
described several ways of preparing antimony sulfide for medical purposes in his treatise ''Natural History'', around 77 AD. Pliny the Elder also made a distinction between "male" and "female" forms of antimony; the male form is probably the sulfide, while the female form, which is superior, heavier, and less friable, has been suspected to be native metallic antimony.
The Greek naturalist Pedanius Dioscorides
Pedanius Dioscorides (, ; 40–90 AD), "the father of pharmacognosy", was a Greek physician, pharmacologist, botanist, and author of (in the original , , both meaning "On Medical Material") , a 5-volume Greek encyclopedic pharmacopeia on he ...
mentioned that antimony sulfide could be roasted by heating by a current of air. It is thought that this produced metallic antimony.
Antimony was frequently described in alchemical manuscripts, including the ''Summa Perfectionis'' of Pseudo-Geber
Pseudo-Geber (or "Middle Latin, Latin pseudo-Geber") is the presumed author or group of authors responsible for a corpus of pseudepigraphic alchemical writings dating to the late 13th and early 14th centuries. These writings were falsely attrib ...
, written around the 14th century. A description of a procedure for isolating antimony is later given in the 1540 book '' De la pirotechnia'' by Vannoccio Biringuccio, predating the more famous 1556 book by Agricola
Agricola, the Latin word for farmer, may also refer to:
People Cognomen or given name
:''In chronological order''
* Gnaeus Julius Agricola (40–93), Roman governor of Britannia (AD 77–85)
* Sextus Calpurnius Agricola, Roman governor of the m ...
, '' De re metallica''. In this context Agricola has been often incorrectly credited with the discovery of metallic antimony. The book ''Currus Triumphalis Antimonii'' (The Triumphal Chariot of Antimony), describing the preparation of metallic antimony, was published in Germany in 1604. It was purported to be written by a Benedictine
The Benedictines, officially the Order of Saint Benedict (, abbreviated as O.S.B. or OSB), are a mainly contemplative monastic order of the Catholic Church for men and for women who follow the Rule of Saint Benedict. Initiated in 529, th ...
monk, writing under the name Basilius Valentinus in the 15th century; if it were authentic, which it is not, it would predate Biringuccio.
The metal antimony was known to German chemist Andreas Libavius in 1615 who obtained it by adding iron to a molten mixture of antimony sulfide, salt and potassium tartrate. This procedure produced antimony with a crystalline or starred surface.
With the advent of challenges to phlogiston theory
The phlogiston theory, a superseded scientific theory, postulated the existence of a fire-like element dubbed phlogiston () contained within combustible bodies and released during combustion. The name comes from the Ancient Greek (''burnin ...
, it was recognized that antimony is an element forming sulfides, oxides, and other compounds, as do other metals.
The first discovery of naturally occurring pure antimony in the Earth's crust
Earth's crust is its thick outer shell of rock, referring to less than one percent of the planet's radius and volume. It is the top component of the lithosphere, a solidified division of Earth's layers that includes the crust and the upper ...
was described by the Swedish scientist and local mine district engineer Anton von Swab in 1783; the type-sample was collected from the Sala Silver Mine in the Bergslagen mining district of Sala, Västmanland, Sweden.
Etymology
The medieval Latin form, from which the modern languages and lateByzantine Greek
Medieval Greek (also known as Middle Greek, Byzantine Greek, or Romaic; Greek: ) is the stage of the Greek language between the end of classical antiquity in the 5th–6th centuries and the end of the Middle Ages, conventionally dated to the F ...
take their names for antimony, is '. The origin of that is uncertain, and all suggestions have some difficulty either of form or interpretation. The popular etymology, from ἀντίμοναχός ''anti-monachos'' or French , would mean "monk-killer", which is explained by the fact that many early alchemists were monks, and some antimony compounds were poisonous.
Another popular etymology is the hypothetical Greek word ἀντίμόνος ''antimonos'', "against aloneness", explained as "not found as metal", or "not found unalloyed"."Antimony" in ''Kirk-Othmer Encyclopedia of Chemical Technology'', 5th ed. 2004. However, ancient Greek would more naturally express the pure negative as ''α-'' ("not"). Edmund Oscar von Lippmann conjectured a hypothetical Greek word ανθήμόνιον ''anthemonion'', which would mean "floret", and cites several examples of related Greek words (but not that one) which describe chemical or biological efflorescence.Edmund Oscar von Lippmann, von Lippmann, Edmund Oscar (1919) Entstehung und Ausbreitung der Alchemie, teil 1. Berlin: Julius Springer (in German). pp. 642–5
The early uses of ''antimonium'' include the translations, in 1050–1100, by Constantine the African of Arabic medical treatises. Several authorities believe ''antimonium'' is a scribal corruption of some Arabic form; Meyerhof derives it from ''ithmid''; other possibilities include ''athimar'', the Arabic name of the metalloid, and a hypothetical ''as-stimmi'', derived from or parallel to the Greek.
The standard chemical symbol for antimony (Sb) is credited to Jöns Jakob Berzelius, who derived the abbreviation from ''stibium''.
The ancient words for antimony mostly have, as their chief meaning, kohl, the sulfide of antimony.
The Egyptians called antimony ''mśdmt'' or ''stm''.
The Arabic word for the substance, as opposed to the cosmetic, can appear as ''ithmid, athmoud, othmod'', or ''uthmod''. Littré suggests the first form, which is the earliest, derives from ''stimmida'', an accusative for ''stimmi''. The Greek word στίμμι (stimmi) is used by Attica, Attic tragedy, tragic poets of the 5th century BC, and is possibly a loan word from Arabic or from Egyptian ''stm''.
Production
Process
The extraction of antimony from ores depends on the quality and composition of the ore. Most antimony is mined as the sulfide; lower-grade ores are concentrated by froth flotation, while higher-grade ores are heated to 500–600 °C, the temperature at which stibnite melts and separates from the gangue minerals. Antimony can be isolated from the crude antimony sulfide by reduction with scrap iron: : The sulfide is converted to an oxide by roasting. The product is further purified by vaporizing the volatile antimony(III) oxide, which is recovered. This sublimate is often used directly for the main applications, impurities being arsenic and sulfide., [ p. 45] Antimony is isolated from the oxide by a carbothermal reduction: : The lower-grade ores are reduced in blast furnaces while the higher-grade ores are reduced in reverberatory furnaces.Top producers and production volumes
In 2022, according to the US Geological Survey, China accounted for 54.5% of total antimony production, followed in second place by Russia with 18.2% and Tajikistan with 15.5%. Chinese production of antimony is expected to decline in the future as mines and smelters are closed down by the government as part of pollution control. Especially due to an environmental protection law having gone into effect in January 2015 and revised "Emission Standards of Pollutants for Stanum, Antimony, and Mercury" having gone into effect, hurdles for economic production are higher. Reported production of antimony in China has fallen and is unlikely to increase in the coming years, according to the Roskill report. No significant antimony deposits in China have been developed for about ten years, and the remaining economic reserves are being rapidly depleted.Reserves
Supply risk
For antimony-importing regions, such as Europe and the U.S., antimony is considered to be a Critical mineral raw materials, critical mineral for industrial manufacturing that is at risk of supply chain disruption. With global production coming mainly from China (74%), Tajikistan (8%), and Russia (4%), these sources are critical to supply. *European Union: Antimony is considered a critical raw material for defense, automotive, construction and textiles. The E.U. sources are 100% imported, coming mainly from Turkey (62%), Bolivia (20%) and Guatemala (7%). *United Kingdom: The British Geological Survey's 2015 risk list ranks antimony second highest (after Rare-earth element, rare earth elements) on the relative supply risk index. *United States: Antimony is a mineral commodity considered critical to the economic and national security. In 2022, no antimony was mined in the U.S.Applications
Approximately 48% of antimony is consumed inflame retardant
Flame retardants are a diverse group of chemicals that are added to manufactured materials, such as plastics and textiles, and surface finishes and coatings. Flame retardants are activated by the presence of an combustion, ignition source and pr ...
s, 33% in Lead–acid battery, lead–acid batteries, and 8% in plastics.
Flame retardants
Antimony is mainly used as the antimony trioxide, trioxide for flame retardant, flame-proofing compounds, always in combination with halogenated flame retardants except in halogen-containing polymers. The flame retarding effect of antimony trioxide is produced by the formation of halogenated antimony compounds, which react with hydrogen atoms, and probably also with oxygen atoms and OH radicals, thus inhibiting fire. Markets for these flame-retardants include children's clothing, toys, aircraft, and automobile seat covers. They are also added to polyester resins in glass-reinforced plastic, fiberglass composite material, composites for such items as light aircraft engine covers. The resin will burn in the presence of an externally generated flame, but will extinguish when the external flame is removed.Grund, Sabina C.; Hanusch, Kunibert; Breunig, Hans J.; Wolf, Hans Uwe (2006) "Antimony and Antimony Compounds" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim.Alloys
Antimony forms a highly usefulalloy
An alloy is a mixture of chemical elements of which in most cases at least one is a metal, metallic element, although it is also sometimes used for mixtures of elements; herein only metallic alloys are described. Metallic alloys often have prop ...
with lead, increasing its hardness and mechanical strength. When casting it increases fluidity of the melt and reduces shrinkage during cooling. For most applications involving lead, varying amounts of antimony are used as alloying metal. In lead–acid batteries, this addition improves plate strength and charging characteristics. For sailboats, lead keels are used to provide righting moment, ranging from 600 lbs to over 200 tons for the largest sailing superyachts; to improve hardness and tensile strength of the lead keel, antimony is mixed with lead between 2% and 5% by volume. Antimony is used in antifriction alloys (such as Babbitt metal), in bullets and lead shot, electrical cable sheathing, type metal (for example, for Linotype machine, linotype printing machines), solder
Solder (; North American English, NA: ) is a fusible alloy, fusible metal alloy used to create a permanent bond between metal workpieces. Solder is melted in order to wet the parts of the joint, where it adheres to and connects the pieces aft ...
(some "RoHS, lead-free" solders contain 5% Sb), in pewter, and in hardening alloys with low tin content in the manufacturing of organ pipes.
Other applications
 Three other applications consume nearly all the rest of the world's supply. One application is as a stabilizer and catalyst for the production of polyethylene terephthalate. Another is as a fining agent to remove microscopic bubbles in glass, mostly for TV screens antimony ions interact with oxygen, suppressing the tendency of the latter to form bubbles. The third application is pigments.
In the 1990s antimony was increasingly being used in semiconductors as a
Three other applications consume nearly all the rest of the world's supply. One application is as a stabilizer and catalyst for the production of polyethylene terephthalate. Another is as a fining agent to remove microscopic bubbles in glass, mostly for TV screens antimony ions interact with oxygen, suppressing the tendency of the latter to form bubbles. The third application is pigments.
In the 1990s antimony was increasingly being used in semiconductors as a dopant
A dopant (also called a doping agent) is a small amount of a substance added to a material to alter its physical properties, such as electrical or optics, optical properties. The amount of dopant is typically very low compared to the material b ...
in N-type semiconductor, n-type silicon wafer (electronics), wafers for diodes, infrared detectors, and Hall effect, Hall-effect devices. In the 1950s, the emitters and collectors of n-p-n alloy junction transistors were doped with tiny beads of a lead-antimony alloy. Indium antimonide (InSb) is used as a material for mid-infrared detectors.
The material is used as for phase-change memory, a type of computer memory.
Biology and medicine have few uses for antimony. Treatments containing antimony, known as antimonials, are used as emetics. Antimony compounds are used as antiprotozoal agent, antiprotozoan drugs. Potassium antimonyl tartrate, or tartar emetic, was once used as an anti-schistosomiasis, schistosomal drug from 1919 on. It was subsequently replaced by praziquantel. Antimony and its compounds are used in several veterinary preparations, such as anthiomaline and lithium antimony thiomalate, as a skin conditioner in ruminants. Antimony has a nourishing or conditioning effect on keratinized tissues in animals.
Antimony-based drugs, such as meglumine antimoniate, are also considered the drugs of choice for treatment of leishmaniasis. Early treatments used antimony(III) species (trivalent antimonials), but in 1922 Upendranath Brahmachari invented a much safer antimony(V) drug, and since then so-called pentavalent antimonials have been the standard first-line treatment. However, ''Leishmania'' strains in Bihar and neighboring regions have developed resistance to antimony. Elemental antimony as an antimony pill was once used as a medicine. It could be reused by others after ingestion and elimination.
Antimony(III) sulfide is used in the heads of some safety matches. Antimony sulfides help to stabilize the friction coefficient in automotive brake pad materials. Antimony is used in bullets, bullet tracers, paint, glass art, and as an opacifier in Vitreous enamel, enamel. Antimony-124 is used together with beryllium in neutron sources; the gamma rays emitted by antimony-124 initiate the photodisintegration of beryllium. The emitted neutrons have an average energy of 24 keV. Natural antimony is used in startup neutron sources.
The powder derived from crushed antimony sulfide ('' kohl'') has been used for millennia as an eye cosmetic. Historically it was applied to the eyes with a metal rod and with one's spittle, and was thought by the ancients to aid in curing eye infections. The practice is still seen in Yemen and in other Muslim countries.
Precautions
Antimony and many of its compounds are toxic, and the effects of antimony poisoning are similar to arsenic poisoning. The toxicity of antimony is far lower than that of arsenic; this might be caused by the significant differences of uptake, metabolism and excretion between arsenic and antimony. The uptake of antimony(III) or antimony(V) in the gastrointestinal tract is at most 20%. Antimony(V) is not quantitatively reduced to antimony(III) in the cell (in fact antimony(III) is oxidised to antimony(V) instead). Since methylation of antimony does not occur, the excretion of antimony(V) in urine is the main way of elimination. Like arsenic, the most serious effect of acute antimony poisoning is cardiotoxicity and the resulting myocarditis; however, it can also manifest as Adams–Stokes syndrome, which arsenic does not. Reported cases of intoxication by antimony equivalent to 90 mg antimony potassium tartrate dissolved from enamel has been reported to show only short term effects. An intoxication with 6 g of antimony potassium tartrate was reported to result in death after three days. Inhalation of antimony dust is harmful and in certain cases may be fatal; in small doses, antimony causes headaches, dizziness, and depression. Larger doses such as prolonged skin contact may cause dermatitis, or damage the kidneys and the liver, causing violent and frequent vomiting, leading to death in a few days. Antimony is incompatible with strong oxidizing agents, strong acids, hydrohalic acid, halogen acids, chlorine, or fluorine. It should be kept away from heat. Antimony leaching (chemical science), leaches from polyethylene terephthalate (PET) bottles into liquids. While levels observed for bottled water are below drinking water guidelines, fruit juice concentrates (for which no guidelines are established) produced in the UK were found to contain up to 44.7 μg/L of antimony, well above the EU limits for tap water of 5 μg/L. The guidelines are: * World Health Organization: 20 μg/L * Japan: 15 μg/L * United States Environmental Protection Agency, Health Canada and the Ontario Ministry of Environment: 6 μg/LScreening assessment antimony-containing substancesHealth Canada. July 2020. * EU and German Federal Ministry of Environment: 5 μg/L The tolerable daily intake (TDI) proposed by WHO is 6 μg antimony per kilogram of body weight. The immediately dangerous to life or health (IDLH) value for antimony is 50 mg/m3.
Toxicity
Certain compounds of antimony appear to be toxic, particularly antimony trioxide and antimony potassium tartrate. Effects may be similar to arsenic poisoning. Occupational exposure may cause respiratory irritation, pneumoconiosis, antimony spots on the skin, gastrointestinal symptoms, and cardiac arrhythmias. In addition, antimony trioxide is potentially carcinogenic to humans. Adverse health effects have been observed in humans and animals following inhalation, oral, or dermal exposure to antimony and antimony compounds. Antimony toxicity typically occurs either due to occupational exposure, during therapy or from accidental ingestion. It is unclear if antimony can enter the body through the skin. The presence of low levels of antimony in saliva may also be associated with dental decay.Notes
References
Cited sources
* *External links
Public Health Statement for Antimony
International Antimony Association vzw (i2a
Chemistry in its element podcast
(MP3) from the Royal Society of Chemistry's Chemistry World
Antimony
at ''The Periodic Table of Videos'' (University of Nottingham)
CDC – NIOSH Pocket Guide to Chemical Hazards – Antimony
Antimony Mineral data and specimen images
{{Authority control Antimony, Chemical elements Metalloids Native element minerals Nuclear materials Pnictogens Trigonal minerals Minerals in space group 166 Chemical elements with rhombohedral structure