Ankyrins on:
[Wikipedia]
[Google]
[Amazon]
Ankyrins are a family of proteins that mediate the attachment of integral membrane proteins to the spectrin- actin based membrane cytoskeleton. Ankyrins have binding sites for the beta subunit of spectrin and at least 12 families of integral membrane proteins. This linkage is required to maintain the integrity of the
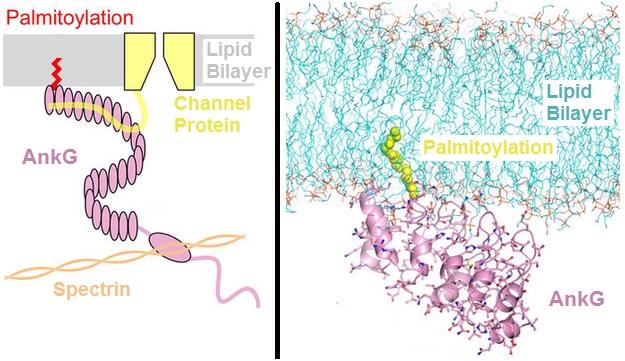 Subsequently, ankyrinB proteins (products of the ANK2 gene) were identified in brain and muscle. AnkyrinB and AnkyrinG proteins are required for the polarized distribution of many membrane proteins including the Na+/K+ ATPase, the voltage gated Na+ channel and the Na+/Ca2+ exchanger.
Subsequently, ankyrinB proteins (products of the ANK2 gene) were identified in brain and muscle. AnkyrinB and AnkyrinG proteins are required for the polarized distribution of many membrane proteins including the Na+/K+ ATPase, the voltage gated Na+ channel and the Na+/Ca2+ exchanger.
plasma membrane
The cell membrane (also known as the plasma membrane (PM) or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of all cells from the outside environment (t ...
s and to anchor specific ion channel
Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of io ...
s, ion exchangers and ion transporter
In biology, a transporter is a transmembrane protein that moves ions (or other small molecules) across a biological membrane to accomplish many different biological functions including, cellular communication, maintaining homeostasis, energy produc ...
s in the plasma membrane. The name is derived from the Greek word ἄγκυρα (''ankyra'') for "anchor".
Structure
Ankyrins contain four functional domains: an N-terminal domain that contains 24 tandem ankyrin repeats, a central domain that binds to spectrin, a death domain that binds to proteins involved inapoptosis
Apoptosis (from grc, ἀπόπτωσις, apóptōsis, 'falling off') is a form of programmed cell death that occurs in multicellular organisms. Biochemical events lead to characteristic cell changes (morphology) and death. These changes incl ...
, and a C-terminal regulatory domain that is highly variable between different ankyrin proteins.
Membrane protein recognition
The 24 tandem ankyrin repeats are responsible for the recognition of a wide range of membrane proteins. These 24 repeats contain 3 structurally distinct binding sites ranging from repeat 1-14. These binding sites are quasi-independent of each other and can be used in combination. The interactions the sites use to bind to membrane proteins are non-specific and consist of: hydrogen bonding, hydrophobic interactions and electrostatic interactions. These non-specific interactions give ankyrin the property to recognise a large range of proteins as the sequence doesn't have to be conserved, just the properties of theamino acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha am ...
. The quasi-independence means that if a binding site is not used, it won't have a large effect on the overall binding. These two properties in combination give rise to large repertoire of proteins ankyrin can recognise.
Subtypes
Ankyrins are encoded by three genes ( ANK1, ANK2 and ANK3) in mammals. Each gene in turn produces multiple proteins throughalternative splicing
Alternative splicing, or alternative RNA splicing, or differential splicing, is an alternative splicing process during gene expression that allows a single gene to code for multiple proteins. In this process, particular exons of a gene may be ...
.
ANK1
The ANK1 gene encodes the AnkyrinR proteins. AnkyrinR was first characterized in human erythrocytes, where this ankyrin was referred to as erythrocyte ankyrin or band2.1. AnkyrinR enables erythrocytes to resist shear forces experienced in the circulation. Individuals with reduced or defective ankyrinR have a form of hemolytic anemia termed hereditary spherocytosis. In erythrocytes, AnkyrinR links the membrane skeleton to the Cl−/HCO3− anion exchanger. Ankyrin 1 links membrane receptor CD44 to theinositol triphosphate receptor
Inositol trisphosphate receptor (InsP3R) is a membrane glycoprotein complex acting as a Ca2+ channel activated by inositol trisphosphate (InsP3). InsP3R is very diverse among organisms, and is necessary for the control of cellular and physiol ...
and the cytoskeleton.
It has been suggested that Ankyrin 1 interacts with KAHRP (shown via selective pull-downs, SPR and ELISA).
ANK2
 Subsequently, ankyrinB proteins (products of the ANK2 gene) were identified in brain and muscle. AnkyrinB and AnkyrinG proteins are required for the polarized distribution of many membrane proteins including the Na+/K+ ATPase, the voltage gated Na+ channel and the Na+/Ca2+ exchanger.
Subsequently, ankyrinB proteins (products of the ANK2 gene) were identified in brain and muscle. AnkyrinB and AnkyrinG proteins are required for the polarized distribution of many membrane proteins including the Na+/K+ ATPase, the voltage gated Na+ channel and the Na+/Ca2+ exchanger.
ANK3
AnkyrinG proteins (products of the ANK3 gene) were identified in epithelial cells and neurons. A large-scale genetic analysis conducted in 2008 shows the possibility that ANK3 is involved in bipolar disorder.See also
*DARPin DARPins (an acronym for designed ankyrin repeat proteins) are genetically engineered antibody mimetic proteins typically exhibiting highly specific and high-affinity target protein binding. They are derived from natural ankyrin repeat proteins, one ...
(designed ankyrin repeat protein), an engineered antibody mimetic based on the structure of ankyrin repeats
References
External links
* * Ankyrin-R {{Cytoskeletal proteins Peripheral membrane proteins Biology of bipolar disorder it:Anchirina